-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(2): 67-73
doi:10.5923/j.fph.20140402.08
Supercritical Fluid Extraction of Beta-ecdysone from Brazilian Ginseng (Pfaffia glomerata) Roots
Isabel C. N. Debien, M. Angela A. Meireles
LASEFI/DEA (Department of Food Engineering) / FEA (School of Food Engineering) / UNICAMP (University of Campinas), Rua Monteiro Lobato, 80; Campinas, SP; CEP: 13083-862, Brazil
Correspondence to: M. Angela A. Meireles, LASEFI/DEA (Department of Food Engineering) / FEA (School of Food Engineering) / UNICAMP (University of Campinas), Rua Monteiro Lobato, 80; Campinas, SP; CEP: 13083-862, Brazil.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Ecdysteroids have been found in a variety of plants and have several valuable biological properties. Beta-ecdysone is the major biologically active ecdysteroid that can be isolated from Pfaffia glomerata roots. Pfaffia glomerata is a medicinal plant known in Brazil as “Brazilian ginseng,” and this plant has been used as a substitute for “Asian” ginseng due their similar morphologies and bioactive properties. In this work, supercritical fluid extraction (SFE) using carbon dioxide (CO2) was used to obtain beta-ecdysone-rich extracts from Brazilian ginseng roots. The effects of pressure (20 and 30 MPa) and cosolvent amount (10, 15, 75 and 90% of Ethanol, EtOH) on the behavior of the overall extraction curve (OEC) and beta-ecdysone content were studied. Larger amounts of beta-ecdysone were obtained in shorter processing times using CO2: EtOH (85:15, v/v) as the extracting solvent at 20 MPa. Higher amounts of EtOH in the solvent mixture leads to increased extraction yield as well as increased beta-ecdysone content.
Keywords: Pfaffia glomerata, Brazilian ginseng roots, Supercritical fluid extraction, Carbon dioxide, Ethanol, Beta-ecdysone
Cite this paper: Isabel C. N. Debien, M. Angela A. Meireles, Supercritical Fluid Extraction of Beta-ecdysone from Brazilian Ginseng (Pfaffia glomerata) Roots, Food and Public Health, Vol. 4 No. 2, 2014, pp. 67-73. doi: 10.5923/j.fph.20140402.08.
Article Outline
1. Introduction
- Ecdysteroids are steroid hormones that are found in insects. Ecdysteroids have also been discovered in plants, and this discovery has initiated fruitful research. Thus far, ecdysteroids have been detected in over 120 plant families. In plants, the amount of ecdysteroids is an order of magnitude higher than in insects [1]. Plants and insects rarely have the same ecdysteroids; therefore, these bioactive compounds play different roles depending on their source. In insects, it is known that these steroids are present at all stages of development, regulating many biochemical and physiological process, whereas in plants, the function of ecdysteroids is still unknown. Ecdysteroids from plants, known as phytoecdysteroids, are apparently non-toxic to mammals and may have a number of beneficial pharmacological and medicinal applications [2]. The presence of phytoecdysteroids has been found in only approximately 2% of the world’s flora; approximately 5-6% of the species that have been tested have phytoecdysteroids, making up approximately 0.1% of the plant’s dry mass. Among the phytoecdysteroids, beta- ecdysone (Figure 1) is recognized as the major biologically active ecdysteroid in most invertebrate systems [3].Beta-ecdysone is the major biologically active ecdysteroid isolated from Pfaffia glomerata roots, a species that belongs to the Amaranthacae family [4]. Pfaffia glomeratais a medicinal plant known in Brazil as “Brazilian ginseng” and as “Suma” elsewhere; ithas been used as a substitute for “Asian” ginseng due their similar morphologies and bioactive properties. However, “Asian” and “Brazilian” ginseng have different compositions, and ecdysteroids are present only in the latter genus. Beta-ecdysone is used as a good chemical marker for raw material quality due its adaptogen effect [5]. The term adaptogen is used to classify a group of substances that can improve the body´s nonspecific resistance after being exposed to various stressing factors, promoting a state of adaptation to the exceptional situation [6].Several pharmacological and medicinal studies have demonstrated that Brazilian ginseng roots (BGR) extracts have potential analgesic, anti-inflammatory [7], gastroprotective [8], antinociceptive [9], anti-glycemic [10] and anti-microbial [11] properties. Additional studies have shown that BGR extracts may also act as a melanogenesis inhibitor [12] and as a central nervous system depressant [13]. Therefore, considering the health-promoting attributes of BGR extracts, their use as a food additive has great potential for nutraceutical food production. Nonetheless, beta-ecdysone might have estrogenic properties or activities and can cause an increase in estrogen production as this plant has been used traditionally to regulate menstrual processes, as well as to treat menopause and other hormonal disorders [14]. There are some studies of beta-ecdysone showing significant anabolic effects [15, 16]. It is not a testosterone booster, but has a potential as an ergogenic aid. All those studies that report this benefit has limited details available to evaluate the experimental design. Thus is early to tell whether beta-ecdysone serve as safe and effective nutritional supplement for athletes [17]. Nevertheless, it is necessary more studies about anabolic and toxicity effects of beta-ecdysone. There were reported two weaknesses of BGR, the BGR powder can cause asthmatic allergic reactions if inhaled, thus when handling or preparing the BGR avoid inhalation of the powder. The other weakness is that the ingestion of large amounts of saponins (naturally occurring chemicals in BGR) has shown to sometimes cause mild gastric disturbances including nausea and stomach cramping [4]. Therefore the dosages control is so important.Extracts from several botanic matrices have found use in the health and food fields, mainly due their antioxidant properties. These botanic matrices include rice bran [18] [14], annatto [19], turmeric [20] and also BGR [21].In addition, some Brazilian pharmaceutical manufacturers that produce phytopharmaceuticals and food supplements containing micronized roots and/or extracts of BGR report beta-ecdysone as the active compound [22].Commercially, extracts from BGR-containing bioactive compounds are obtained by conventional solid-liquid extraction methods, which use large quantities of extracting solvents [23]. Currently, more environmentally friendly methods, such as supercritical fluid extraction (SFE), are preferred. SFE has been successfully used to extract several bioactive compounds from different plants [24].SFE using carbon dioxide (CO2) as a solvent is an attractive alternative method for compound recovery because it is an environment safe process that yields high quality products. SFE has several advantages over conventional methods. One of the main characteristics of SFE is that changing the operating conditions allows the selective recovery of compounds without toxic residues [25].
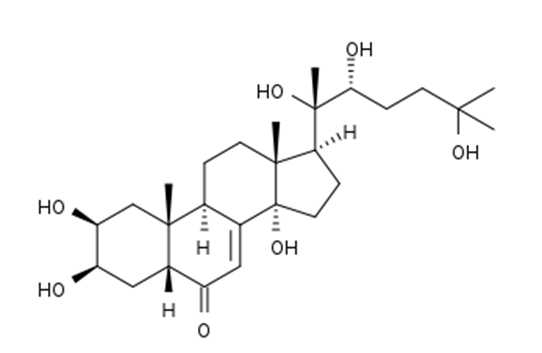 | Figure 1. Chemical structure of beta-ecdysone |
2. Materials and Methods
2.1. Raw Material Characterization and Preparation
- BGR were cultivated in the experimental field of CPQBA (Campinas, Brazil) and were collected on November 17, 2008, after 7 years of cultivation. BGR were washed and dried in a forced air circulation dryer at 313 K for 5 days. The dried roots (8.9% moisture) were then comminuted in a pulse mill (Marconi, model MA 340, Piracicaba, Brazil) for a few seconds. Next, larger particles were milled a second time with a knife mill (Tecnal, model TE 631, Piracicaba, Brazil) for 2 s at 18,000rpm. Particles were then separated by size using sieves (Series Tyler, W.S. Tyler, Wheeling, USA). The milled BGR were stored in a freezer (Metalfrio, model DA 420, São Paulo, Brazil) at 263 K. For extraction assays, particles 8 µm in diameter, determined according to the ASAE methodology [30], were used. The moisture content of the dried roots was determined by the AOAC method (Method 4.1.03) [31].
2.2. Experimental Design
- The temperature for all assays was 333 K. The effects of the following parameters on the OECs and beta-ecdysone content in the BGR extracts were evaluated: pressure (20 and 30 MPa) and solvent mixture of CO2 and ethanol (EtOH) (%, v/v). The experimental extraction conditions that were evaluated are shown in Table 1.
|
2.3. Extraction Procedure
- A kinetic study was conducted using the SFE system shown in Figure 2 and described in detail Veggi et al. [32]. In short, the 415 cm3 extraction cell was heated by a jacket connected to a thermostatic bath until the desired temperature was reached. The supercritical solvent, CO2, was pumped by a pneumatic pump (Maximator, model PP 111-VE MBR, Nordhausen, Germany) and the cosolvent, EtOH, was pumped by an HPLC pump (Thermoseparation Products, Model Consta Metric 3200 P/F, Florida, USA). Approximately 20 grams of BGR was placed in the extraction cell. The sample occupied approximately 4.4% of the cell’s volume, and the empty space of the cell was filled with a Teflon column. After pressurization, the mixture of BGR, supercritical carbon dioxide and EtOH was kept statically at the desired pressure for 30 minutes (static time). Thereafter, the block valve (Autoclave engineers, Model 10V2071 15000psi, Erie, USA) was opened, and the pressure was kept constant with the help of a backpressure valve (TESCOM model 2617-6-1-2-2-065, Elk River, USA) and a heated micrometric valve (Autoclave engineers, Modelo 10VRMM 11000PSI Erie, USA).
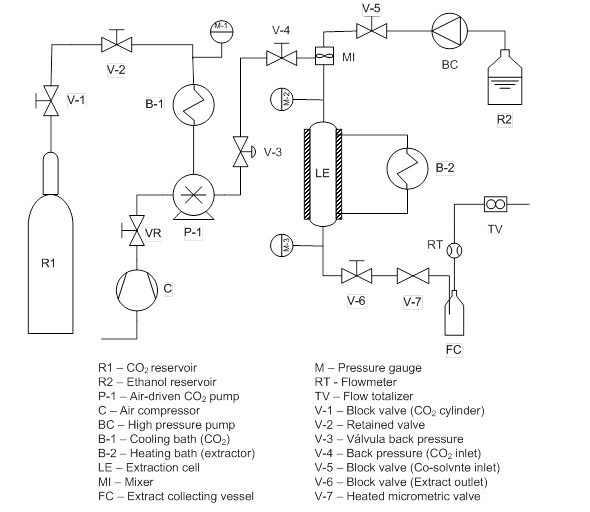 | Figure 2. Schematic diagram of the supercritical CO2 extraction unit (adapted to [28]) |
2.4. Beta-ecdysone Quantification
- Beta-ecdysone quantification was performed according to the methodology described by Leal et al. [26] with modifications. The HPLC Agilent 1260 (Santa Clara, USA) system that was used included a quaternary pump (G1311A), photodiode array detector (G4214B), column oven (G1311A) and automatic injector (G1329B). A Zorbax Eclipse Plus column (C18, 5 µm, 150 × 4.6 mm, Agilent, Santa Clara, USA) at 323 K was used. The mobile phases were water with acetic acid 0.1% (solvent A) and acetonitrile with acetic acid 0.1% (solvent B). Separation was achieved using a linear gradient of solvent B from 0 to 100% in 15 min and then from 100 to 0% in 10 min. Finally, solvent A was maintained at 100% for 5 min at a flow rate of 1.2 cm3.min-1. Peaks were identified by comparing their retention times and UV-vis spectra with those of known standards. Quantification was based on peak area measurements at 246 nm. The linearity of the method was determined using a 20-hydroxyecdysone (beta-ecdysone) standard (≥ 93%, Sigma). Dried extracts were diluted in 80% methanol to obtain a solution with a concentration of 10 mg.cm-3, and the solution was filtered through a 0.45-µm membrane (Millipore) prior to injection.
3. Results and Discussion
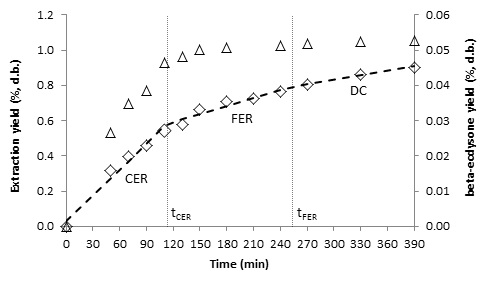
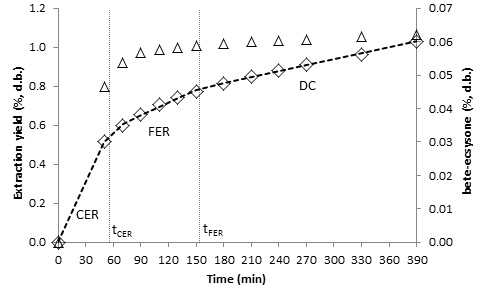
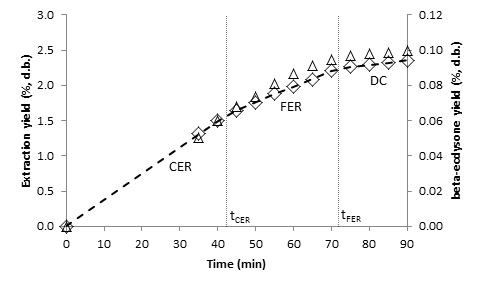
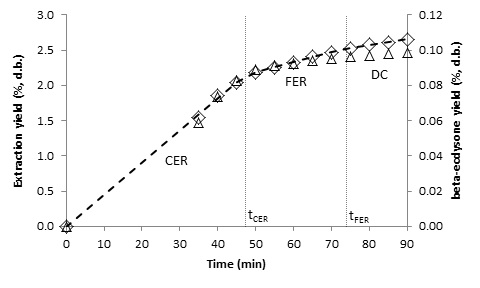
- The kinetics of the SFE process can be characterized empirically by OECs, and kinetic parameters can be calculated by fitting the experimental data to a linear spline [18]. Figures 3, 4, 5 and 6 show the OECs of BGR for different conditions. The three periods of a typical OEC [24] can be observed in the figures. The first line represents the constant extraction rate (CER) period, while the second line represents the falling extraction rate (FER) period. The third line represents the diffusion-controlled rate (DC) period. The duration of the CER period (tCER) is determined by the interception of the first and second lines, and the duration of the FER period (tFER) is determined by the interception of the second and third lines [18].
4. Conclusions
- Supercritical fluid extraction with carbon dioxide and ethanol as a cosolvent is an alternative to organic solvent extraction for generating beta-ecdysone-rich extracts from BGR. Larger amounts of beta-ecdysone were obtained with shorter processing times using CO2: EtOH (85:15, v/v) as the extracting solvent at 20 MPa. A higher proportion of EtOH in the mixture leads to an increase in extraction yield and an increase in beta-ecdysone content relative the raw material amount. These results show that EtOH is more selective for beta-ecdysone recovery than CO2 for beta-ecdysone extraction from BGR.Among the extraction conditions studied, the best condition in terms of beta-ecdysone recovery from BGR was 20 MPa with CO2: EtOH (10:90, v/v) as the extracting solvent. These conditions produce approximately 82% of the total extraction yield and 89% of the total beta-ecdysone content after approximately one hour of extraction.A scale-up and optimization study could further shorten the process time and increase yields of bioactive compounds.
ACKNOWLEDGEMENTS
- Isabel C. N. Debien thanks CNPq for the Ph.D. assistantship (151165/2010-6). M. A. A. Meireles acknowledges the productivity grant (301301/2010-7) from CNPq. The authors acknowledge the financial support from CNPq and FAPESP (2009/17234-9; 2012/10685-8).
References
| [1] | M. Báthori, "Purification and characterization of plant ecdysteroids of Silene species", TrAC Trends in Analytical Chemistry, vol.17, no.6, pp.372-383, 1998. |
| [2] | R. A. Festucci-Buselli, L. A. Contim, L. C. A. Barbosa, J. J. Stuart, R. F. Vieira, W. C. Otoni, "Level and distribution of 20-hydroxyecdysone during Pfaffia glomerata development", Brazilian Journal of Plant Physiology, vol.20, no.4, pp.305-311, 2008. |
| [3] | L. Dinan, "Phytoecdysteroids: biological aspects", Phytochemistry, vol.57, no.3, pp.325-339, 2001. |
| [4] | Y. Shiobara, S.-S. Inoue, K. Kato, Y. Nishiguchi, Y. Oishi, N. Nishimoto, F. de Oliveira, G. Akisue, M. K. Akisue, G. Hashimoto, "A nortriterpenoid, triterpenoids and ecdysteroids from Pfaffia glomerata", Phytochemistry, vol.32, no.6, pp.1527-1530, 1993. |
| [5] | A. R. Zimmer, F. Bruxel, V. L. Bassani, G. Gosmann, "HPLC method for the determination of ecdysterone in extractive solution from Pfaffia glomerata", Journal of Pharmaceutical and Biomedical Analysis, vol.40, no.2, pp.450-453, 2006. |
| [6] | F. R. Mendes, "Tonic, fortifier and aphrodisiac: adaptogens in the Brazilian folk medicine", Revista Brasileira de Farmacognosia, vol.21, no.4, pp.754-763, 2011. |
| [7] | A. G. Neto, J. M. L. C. Costa, C. C. Belati, A. H. C. Vinhólis, L. S. Possebom, A. A. Da Silva Filho, W. R. Cunha, J. C. T. Carvalho, J. K. Bastos, M. L. A. e Silva, "Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen", Journal of Ethnopharmacology, vol.96, no.1-2, pp.87-91, 2005. |
| [8] | C. S. Freitas, C. H. Baggio, J. E. Da Silva-Santos, L. Rieck, C. A. de Moraes Santos, C. C. Júnior, L. C. Ming, D. A. Garcia Cortez, M. C. A. Marques, "Involvement of nitric oxide in the gastroprotective effects of an aqueous extract of Pfaffia glomerata (Spreng) Pedersen, Amaranthaceae, in rats", Life Sci., vol.74, no.9, pp.1167-1179, 2004. |
| [9] | C. S. Freitas, C. H. Baggio, A. Twardowschy, A. C. d. Santos, B. Mayer, A. P. Luiz, C. A. M. d. Santos, M. C. A. Marques, A. R. S. d. Santos, "Involvement of glutamate and cytokine pathways on antinociceptive effect of Pfaffia glomerata in mice", Journal of Ethnopharmacology, vol.122, no.3, pp.468-472, 2009. |
| [10] | N. R. Sanches, R. Galletto, C. E. Oliveira, R. B. Bazotte, D. A. G. Cortez, "Avaliação do potencial anti-hiperglicemiante de Pfaffia glomerata (Spreng.) Pedersen (Amaranthaceae)", Acta Scientiarum, vol.23, no.2, pp.613-617, 2001. |
| [11] | A. G. Neto, A. A. da Silva Filho, J. M. L. C. Costa, A. H. C. Vinholis, G. H. B. Souza, W. R. Cunha, M. L. A. E. Silva, S. Albuquerque, J. K. Bastos, "Evaluation of the trypanocidal and leishmanicidal in vitro activity of the crude hydroalcoholic extract of Pfaffia glomerata (Amarathanceae) roots", Phytomedicine, vol.11, no.7-8, pp.662-665, 2004. |
| [12] | S. Nakamura, G. Chen, S. Nakashima, H. Matsuda, Y. Pei, M. Yoshikawa, "Brazilian natural medicines. IV. New Noroleanane-type triterpene and ecdysterone-type sterol glycosides and melanogenesis inhibitors from the roots of Pfaffia glomerata", Chem. Pharm. Bull., vol.58, no.5, pp.690-695, 2010. |
| [13] | F. de-Paris, G. Neves, J. B. Salgueiro, J. Quevedo, I. Izquierdo, S. M. K. Rates, "Psychopharmacological screening of Pfaffia glomerata Spreng. (Amarathanceae) in rodents", Journal of Ethnopharmacology, vol.73, no.1-2, pp.261-269, 2000. |
| [14] | Leslie Taylor, Herbal secrets of the rainforest: the healing power of over 50 medicinal plants you should know about, Prima Pub., 1998. |
| [15] | NS Chermnykh, NL Shimanovskiĭ, GV Shutko, VN Syrov, "The action of methandrostenolone and ecdysterone on the physical endurance of animals and on protein metabolism in the skeletal muscles", Farmakologiia i toksikologiia, vol.51, no.6, pp.57-60, 1987. |
| [16] | Michael D Scheiber, Robert W Rebar, "Isoflavones and postmenopausal bone health: a viable alternative to estrogen therapy?", Menopause, vol.6, no.3, pp.233-241, 1999. |
| [17] | Tyler LeBaron, Testosterone Booster Recommendation Report. Muscle Feast, 2011. |
| [18] | Susana P. Jesus, Maurício N. Calheiros, Haiko Hense, M. Angela A. Meireles, A Simplified Model to Describe the Kinetic Behavior of Supercritical Fluid Extraction from a Rice Bran Oil Byproduct, Food and Public Health, vol. 3, no. 4, 215-222, 2013. |
| [19] | Moyses N. Moraes, Giovani L. Zabot, Juliana M. Prado, M. Angela A. Meireles, "Obtaining Antioxidants from Botanic Matrices Applying Novel Extraction Techniques", Food and Public Health, vol.3, no.4, pp.195-214, 2013. |
| [20] | J. Felipe Osorio-Tobón, M. Angela A. Meireles, "Recent Applications of Pressurized Fluid Extraction: Curcuminoids Extraction with Pressurized Liquids", Food and Public Health, vol.3, no.6, pp.289-303, 2013. |
| [21] | Diego T. Santos, Dayane F. Barbosa, Ketllen Broccolo, M. Thereza M. S. Gomes, Renata Vardanega, M. Angela A. Meireles, "Pressurized organic solvent extraction with on-line particle formation by supercritical anti solvent processes", Food and Public Health, vol.2, no.6, pp.231-240, 2012. |
| [22] | R. Flores, D. Brondani, Jr., V. Cezarotto, Jr., S. Giacomelli, F. Nicoloso, "Micropropagation and β-ecdysone content of the Brazilian ginsengs Pfaffia glomerata and Pfaffia tuberosa", In Vitro Cell.Dev.Biol.-Plant, vol.46, no.2, pp.210-217, 2010. |
| [23] | P. Matsuzaki, G. Akisue, S. C. S. Oloris, S. Górniak, M. L. Z. Dagli, "Effect of Pfaffia paniculata (Brazilian ginseng) on the Ehrich tumor in its ascitic form", Life Sci., vol.74, pp.473-579, 2003. |
| [24] | M. Angela A. Meireles, Extraction of Bioactive Compounds from Latin American Plants, in Supercritical Fluid Extraction of nutraceuticals and bioactive compunds J. Martinez. CRC Press - Taylor & Francis Group, Boca Raton, 2008. |
| [25] | Camila G. Pereira, M. Angela A. Meireles, "Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives", Food Bioprocess Technol., vol.3, no.3, pp.340-372, 2010. |
| [26] | Patrícia F. Leal, Marina B. Kfouri, Fábio C. Alexandre, Fábio H. R. Fagundes, Juliana M. Prado, Marcos H. Toyama, M. Angela A. Meireles, "Brazilian Ginseng extraction via LPSE and SFE: Global yields, extraction kinetics, chemical composition and antioxidant activity", J. Supercrit. Fluids, vol.54, no.1, pp.38-45, 2010. |
| [27] | H.-C. Wang, C.-R. Chen, C. J. Chang, "Carbon dioxide extraction of ginseng root hair oil and ginsenosides", Food Chem., vol.72, no.4, pp.505-509, 2001. |
| [28] | J. A. Wood, M. A. Bernards, W.-k. Wan, P. A. Charpentier, "Extraction of ginsenosides from North American ginseng using modified supercritical carbon dioxide", The Journal of Supercritical Fluids, vol.39, no.1, pp.40-47, 2006. |
| [29] | Diego T. Santos, Renata Vardanega, Juliana Q. Albarelli, Adriano V. Ensinas, François Maréchal, M. Angela A. Meireles, "Energy Consumption Versus Antioxidant Activity of Pressurized Fluid Extracts from Pfaffia glomerata Roots", CHEMIICAL ENGIINEERIING TRANSACTIIONS, vol.35, 2013. |
| [30] | ASAE, Method of Determining and Expressing Fineness of Feed Materials by Sieving, in Americam Society of Agricultural Engineers Standards, Vol. S319.3, 1998. |
| [31] | AOAC, Official methods of analysis of the Association of Official Analytical Chemists, Association of Official Analytical Chemists, 1995. |
| [32] | Priscilla C. Veggi, Juliana M. Prado, Giovana A. Bataglion, Marcos N. Eberlin, M. Angela A. Meireles, "Obtaining phenolic compounds from jatoba (Hymenaea courbaril L.) bark by supercritical fluid extraction", The Journal of supercritical fluids, DOI 10.1016/j.supflu.2014.02.016. |
| [33] | T. Shibuya, T. Ario, S. Fukuda, "Composition", US Patent No. 6224872, 2001. |
| [34] | M. Angela A. Meireles, "Supercritical extraction from solid: process design data (2001–2003)", Current Opinion in Solid State and Materials Science, vol.7, no.4, pp.321-330, 2003. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML