-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(2): 60-66
doi:10.5923/j.fph.20140402.07
Systematic Review on Mycobacterium Bovis as Potential Cause of Tuberculosis to Humans in Ethiopia
Araya Mengistu1, Fikre Enquselassie2
1Faculty of Veterinary Medicine, University of Gondar, Ethiopia
2School of Public Health, Addis Ababa University, Ethiopia
Correspondence to: Araya Mengistu, Faculty of Veterinary Medicine, University of Gondar, Ethiopia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Mycobacterium bovis is a zoonotic pathogen; widely distributed throughout the world having a range of hosts imposing great risk to the public particularly for those who had direct contact with cattle. This manuscript was compiled by reviewing published literatures to know the magnitude/distribution of M.bovis and its public health significance in Ethiopia. Cross-sectional study designs were selected and studies done since 2002-2011 were reviewed. Lesions, milk, sputum, and fine needle aspirate were taken from animals and human, respectively. In this review, 309 and 21 M. bovis isolates were recorded from animal and human samples, respectively. The isolation of M. bovis from human cases in Ethiopia indicates how the agent is a potential risk to the public.
Keywords: Animals, Bovine, Human, Infections, Mycobacterium bovis, Zoonoses
Cite this paper: Araya Mengistu, Fikre Enquselassie, Systematic Review on Mycobacterium Bovis as Potential Cause of Tuberculosis to Humans in Ethiopia, Food and Public Health, Vol. 4 No. 2, 2014, pp. 60-66. doi: 10.5923/j.fph.20140402.07.
Article Outline
1. Introduction
- Mycobacterium bovis (M. bovis) primarily the causative agent of bovine tuberculosis is in the Mycobacterium tuberculosis complex of the family Mycobacteriaceae. Mycobacterium bovis, which have a wide host range including humans, can survive for several months in the environment, particularly in cold, dark and moist conditions. Cattle shed M. bovis in respiratory secretions, feces and milk, and sometimes in the urine, vaginal secretions or semen and it can infect humans, primarily by the ingestion of unpasteurized dairy products, inhalation of aerosols and through breaks in the skin. The possibility of transmission of this Mycobacterium to humans from infected animals could be high in areas where there is close contact between human and animals. M. bovis caused as much as 25% of cases of human tuberculosis (TB) in developed countries in the late 19th and early 20th centuries. Today, only 1%–2% of human TB cases in developed countries are caused by M. bovis [1] which usually affects persons who acquired the infection locally before the implementation of control measures or in developing countries where control measures have not been implemented.Mycobacterium bovis / bovine tuberculosis results with worldwide annual losses to agriculture of $3 billion, but the human burden of tuberculosis caused by the agent is still unknown [2]. Some African countries like Cameroon, Djibouti, Egypt, Ghana, Nigeria, Tanzania and Uganda collected 1475 (aggregate) sputum samples from human cases at different health institutes in their respective countries and were able to isolate 43 Mycobacterium bovis which accounts for about 2.9% [3-14]. In Ethiopia, the agent is isolated from human sputum and fine needle aspirates of cervical lymphadenitis samples, which is an indication of the contribution of this agent to human tuberculosis [15-17]. Moreover, the skin test positivity of cattle owned by tuberculosis patients is higher compared to non-tuberculosis household cattle [16]. In general, cattle harboring both M. tuberculosis and M. bovis at a time could put a challenge in the move of tuberculosis prevention/control program that currently runs in our country like the STOP-TB program agenda and the Millennium development goal achievements. This systematic review was aimed to show the magnitude/distribution of M. bovis and its public health importance in Ethiopia.
2. Methodology
- For this review Endnote [18] and Google scholar engines were used to search relevant articles that are done in our country. The study design selected for this purpose was cross-sectional ones since most of the studies conducted so far are based on intradermal tuberculin skin test and meat inspections on animals. In a similar manner, human samples were collected once. In the articles reviewed, isolated M. bovis were identified by culturing and then using either biochemical tests or molecular techniques to confirm the agent. For this review key words; namely, Mycobacterium bovis, M. bovis, Bovine tuberculosis, Cattle tuberculosis, bovine tuberculosis in wildlife and M. bovis in humans were used to search articles using Endnote and Google. As inclusion criteria, culture positivity/biochemical tests, molecular positive results, cross-sectional study designs, human and cattle as well as wildlife samples and Articles written in English languages only were used for this purpose. The review covered research works from 2002-2011.
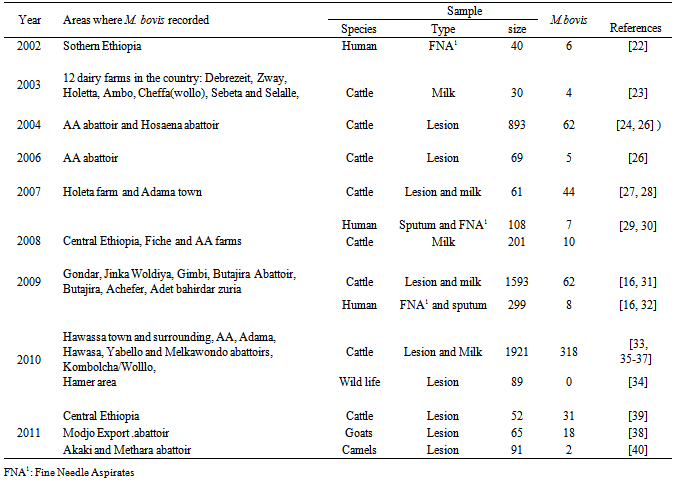
3. Results
- From this review Fine needle aspirate and sputum from humans and milk and tubercle lesions from cattle were used as a sample for M. bovis isolation. Two, 3, 5 and 8 articles revealed the detection of M. bovis from sputum, fine needle aspirate, milk and tubercle lesions, respectively. In some circumstances, two or more samples were collected from a case. The details are presented hereunder. M. bovis, the cause of bovine tuberculosis, was recorded in different parts of the country from human and animal samples. The result is presented in Table-1 based on years of publication. According to this review M. bovis, was reported in four regions of the country from FNA, sputum, milk, lesion samples collected and processed from humans and animal species, respectively. The number of M. bovis isolated from each samples vary and this is presented as a summary in Table-2. From human FNA samples collected and processed M. bovis accounted for about 2.42%, 17.14% and 50% from 165, 35 and 6 Mycobacterium species isolated, respectively.
|
|
|
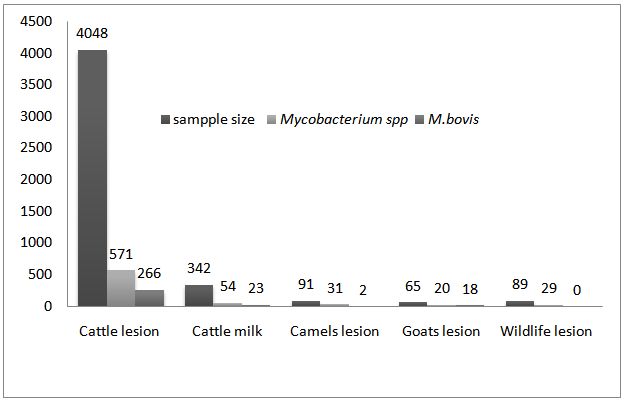 | Figure 1. M. bovis isolated from samples of different animal species |
4. Discussion
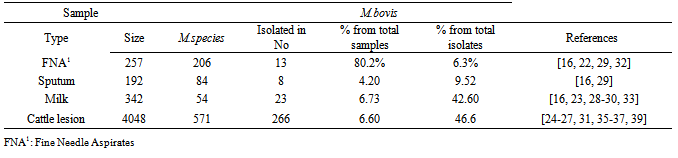
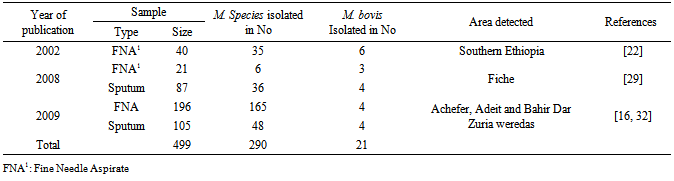
- Mycobacterium bovis, which is a primary cause of bovine tuberculosis, is isolated in samples taken from animals as well as human tuberculosis patients in different parts of the world. Although the exact prevalence of bovine tuberculosis is not known in Ethiopia, different studies showed that the disease is endemic having a range of prevalence values from 10.1% in smallholder farms to 50% in peri-urban intensive dairy farms [19-21]. In our country, samples were collected from animals as well as humans. According to the studies conducted so far M. bovis isolated in 4 regions of the country namely; Amhara National Regional State (Gondar, Woldiya, Adet. Achefer, Bahir Dar zuria, and Kombolcha,Cheffa), Oromiya National Regional State (Central Ethiopia, Fiche, Adama, Methara abattoir, Modjo abattoir, Yabello, Gimbi, Ambo, Selalle, Debrezeit, and Holeta), Southern Nation and Nationalities Regional State (Hawassa, Hamer, Butajira, Hosenna and Melka Wonde) and Addis Ababa [16-40]. Even though the studies conducted are not exhaustive and wide in their scale the results obtained are indicative of the potential of the agent as a risk to the public. The agent was isolated from goats tuberculous lesions collected from Modjo export abattoir at a higher percentage that is, 18 out of 20 (90%) [38]. Nowadays small ruminates like Goat meat are exported to different countries from Ethiopia. The finding of M. bovis in these animals during meat inspection may result in trade embargo from importing countries. In addition to this the isolation of M. bovis from goats indicates that goats could be among the very susceptible host for the agent serving as a source of infection or maintenance host for the agent like badgers (Meles meles) in UK and African Buffalo in Africa [41]. This might need a special attention while considering/conducting any intervention program. In Nigeria out of 1387 screened goats, 62 (4.47%) had tuberculous lesions in their liver, lungs as well as mesenteric lymph nodes and of these 4 goats were confirmed positive for M. bovis by molecular techniques and this figure is very low when compared to the findings in our country [42]. In this review from a total of 4390 milk and lesion samples collected from cattle 289 (6.6%) M. bovis was isolated shown that cattle are the main sources of infection with the agent. In total during these study periods (2002-2011) 4635 samples were collected from different animal species and of these 309 (6.7%) were M. bovis. From the total 705 Mycobacterium species isolated M. bovis accounted for about 43.3% signifying that it is the major isolate in animals [16, 23-31, 33, 35-40] Although the number of isolates was smaller that is 2 out of 31 (6.5%), M. bovis was also isolated from camel samples [40] indicating its importance in this animal species too as well as the agent epidemiology. Besides, eighty nine samples were collected and processed from different wild animal species in Hamer, Southern Ethiopia region. Although M. bovis is reported in wildlife by a number of articles in the literature, the M. bovis was not isolated from wildlife samples. In the review out of 257 Fine Needle Aspirates processed [16, 26, 29]: 206 (80.2%) Mycobacteria species were isolated and 13 (6.3%) of them were M. bovis. From a total of 192 sputum samples processed from human TB cases [16, 29]: 84 (43.8%) Mycobacterium species were isolated and 8 (9.5%) of them were identified as M. bovis. Of the total (290) positive Mycobacteria species isolated in human cases from both samples ([16, 26, 29]) M. bovis accounted for about 7.24%. According to the review M. bovis in humans was isolated in central Ethiopia, Fitche and three West Gojjam district. Isolation of M. bovis from these samples entails the contribution of the agent in pulmonary and extra -pulmonary tuberculosis. Infection of humans with M. bovis and becoming a tuberculosis patient is common in the world. In the United States and in other industrialized nations where few cattle are infected and milk is pasteurized, M. bovis causes less than 1% of tuberculosis cases in humans. From 2001 to 2004 there have been 35 identified cases of M. bovis tuberculosis in New York City [43]. In seven African countries between 2001-2008, from a total of 1475 positive Mycobacterial tuberculosis complexes isolated from suspected tuberculosis human samples 43 (~3. 0%) isolate were M. bovis [3-14]. The figure obtained in these African countries is smaller than the findings obtained in Ethiopia, which is 7.24%. In another scenario in America, in Mercy Hospital, California, from 2000 through 2007, a total of 110 cases of adult HIV-TB co-infection were identified, of which 86 patients had culture-confirmed TB due to M. tuberculosis or M. bovis. Of the 86 TB cases 30 (34.9%) were identified as M. bovis, which is a larger figure compared to us and this might be related to the co-infection with HIV/AIDS in which one accelerates the other [44]. All these studies in general indicate that Bovine tuberculosis is becoming increasingly important due to the susceptibility of humans to the disease/disease causing agent, M. bovis and hence M. bovis infections may be much more significant than generally considered [45].Out of the total 342 cow’s milk samples processed [16, 23, 28-30, 33]; 54 (15.8%) samples revealed a positive result for Mycobacterium species and of which 23/54 (42.6%) were M. bovis with a mean percentage value of 1.12% and 7.10% from the total samples and total isolates, respectively. Similarly, out of the total 4048 different cattle lesion samples processed [24-27, 31, 35, 36, 38] 571 (14.12%) were positive for Mycobacterium species and of which 266/571 (46.60%) were identified as M. bovis with a mean percentage value of o.83% and 5.83% of the total samples and total isolates, respectively. The isolation of M. bovis in both samples was high and this implies that milk and organs could serve as a good source/vehicle of infection for humans as well as animals. Milk should be seen as a vehicle for M. bovis infection to humans since one percent of skin test reactor cows will excrete tubercle bacilli in their milk [46] particularly in countries where bovine tuberculosis remain uncontrolled and ingestion of contaminated raw milk or other dairy products like cheese [47] with M. bovis is practiced. This in fact should be given attention since our community used cheese prepared from raw milk. In general it is possible to say that apart from acquiring M. bovis infections the chance of being a person developing the disease (human bovine tuberculosis) seems realistic and this is confirmed in countries like Egypt, Nigeria, Zaire and Tanzania [48-51]. In a nut shell isolation of M. bovis from these populations would have a great epidemiological importance. In countries like Ethiopia where most of the population lives in the rural community and their lives depend on agriculture, which is mainly, supported by participation of livestock activities the causative agent would have a dual effect to the community. For one thing, the agent may cause diseases to their animals and result in considerable morbidity and mortality thereby reducing labor, production as well as productivity. Secondly, animals would serve as a source of infection for humans to acquire human tuberculosis due to M. bovis and this is the most challenging effect. In countries like ours where animals and humans share the same shelter aerosol transmission could occur effectively. Besides, there is a tradition of taking raw milk in different parts of the country, which could expose individuals to acquire the infection easily and this is particularly true for children whose immunity is not well developed and HIV/AIDS patients whose immunity is compromised.
5. Conclusions and Recommendations
- Although the studies conducted are limited, the current review gives brief evidence on the distribution of M. bovis in different animal species level including human beings and areas in the country. The isolation of the agent from humans indicates the possibility of the agent adaptation to humans having a chance of disease induction by its own stand and therefore it should be seen as a potential cause for human tuberculosis of bovine origin. The agent isolation from camels as well as goats in our country gives an alarming situation as to the circulation of the agent in different animal species indicating the potential danger of the agent to humans, other domestic and wild animals. Even though the agent is not isolated in wild animals as per this review, particularly in areas where there is a communal grazing and watering points for domestic and wild animal populations, the agent transmission would be simplified, thereby complicating its epidemiology. The isolation of M. bovis should be considered as a potential threat to humans in the country and various aspects of the disease should be studied.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML