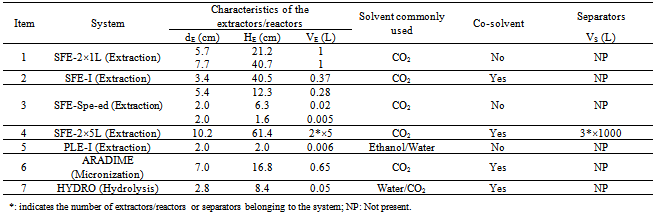
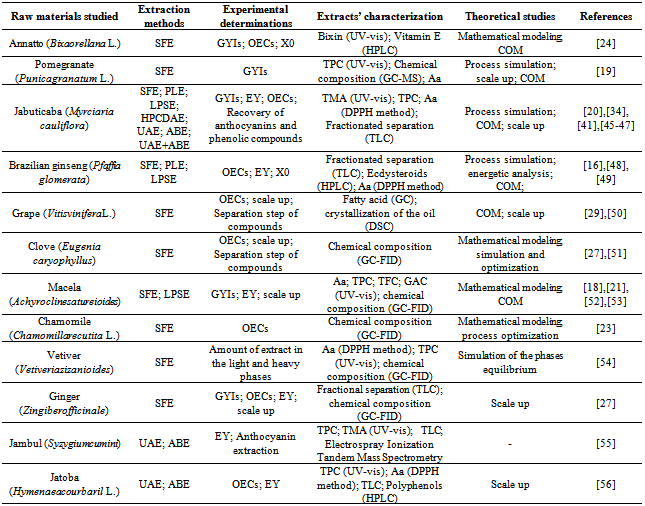
| [1] | M. N. Moraes, G. L. Zabot, J. M. Prado, M. A. A. Meireles, "Obtaining Antioxidants from Botanic Matrices Applying Novel Extraction Techniques", Food and Public Health, vol.3, no.4, pp.195-214, 2013. |
| [2] | G. A. Núñez, C. A. Gelmi, J. M. del Valle, "Simulation of a supercritical carbon dioxide extraction plant with three extraction vessels", Computers and Chemical Engineering, vol.35, no.12, pp.2687-2695, 2011. |
| [3] | D. A. Oliveira, A. A. Salvador, A. Smânia, E. F. A. Smânia, M. Maraschin, S. R. S. Ferreira, "Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids", Journal of Biotechnology, vol.164, no.3, pp.423-432, 2013. |
| [4] | G. Brunner, N. T. Machado, "Process design methodology for fractionation of fatty acids from palm fatty acid distillates in countercurrent packed columns with supercritical CO2", Journal of Supercritical Fluids, vol.66, pp.96-110, 2012. |
| [5] | E. Kiran, "Foaming strategies for bioabsorbable polymers in supercritical fluid mixtures. Part I. Miscibility and foaming of poly(l-lactic acid) in carbon dioxide + acetone binary fluid mixtures", Journal of Supercritical Fluids, vol.54, no.3, pp.308-319, 2010. |
| [6] | J. P. S. Queiroz, M. D. Bermejo, M. J. Cocero, "Kinetic model for isopropanol oxidation in supercritical water in hydrothermal flame regime and analysis", Journal of Supercritical Fluids, vol.76, pp.41-47, 2013. |
| [7] | S. Machmudah, Wahyudiono, N. Takada, H. Kanda, K. Sasaki, M. Goto, "Fabrication of gold and silver nanoparticles with pulsed laser ablation under pressurized CO2", Advances in Natural Sciences: Nanoscience and Nanotechnology, vol.4, no.4, 2013. |
| [8] | D. Villanueva Bermejo, E. Ibáñez, R. P. Stateva, T. Fornari, "Solubility of CO2 in ethyl lactate and modeling of the phase behavior of the CO2 + ethyl lactate mixture", Journal of Chemical and Engineering Data, vol.58, no.2, pp.301-306, 2013. |
| [9] | V. Marulanda, G. Bolaños, "Supercritical water oxidation of a heavily PCB-contaminated mineral transformer oil: Laboratory-scale data and economic assessment", Journal of Supercritical Fluids, vol.54, no.2, pp.258-265, 2010. |
| [10] | H. Mhemdi, E. Rodier, N. Kechaou, J. Fages, "A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds", Journal of Food Engineering, vol.105, no.4, pp.609-616, 2011. |
| [11] | H. Machida, M. Takesue, R. L. Smith, "Green chemical processes with supercritical fluids: Properties, materials, separations and energy", Journal of Supercritical Fluids, vol.60, pp.2-15, 2011. |
| [12] | J. W. King, "Supercritical fluid-based extraction/processing: Then and now", International News on Fats, Oils and Related Materials, vol.23, no.2, pp.124-128, 2012. |
| [13] | D. Ciftci, M. D. A. Saldaña, "Enzymatic synthesis of phenolic lipids using flaxseed oil and ferulic acid in supercritical carbon dioxide media", Journal of Supercritical Fluids, vol.72, pp.255-262, 2012. |
| [14] | K. Gairola, I. Smirnova, "Hydrothermal pentose to furfural conversion and simultaneous extraction with SC-CO2 - Kinetics and application to biomass hydrolysates", Bioresource Technology, vol.123, pp.592-598, 2012. |
| [15] | S. Kazemi, V. Belandria, N. Janssen, D. Richon, C. J. Peters, M. C. Kroon, "Solubilities of ferrocene and acetylferrocene in supercritical carbon dioxide", Journal of Supercritical Fluids, vol.72, pp.320-325, 2012. |
| [16] | P. F. Leal, M. B. Kfouri, F. C. Alexandre, F. H. R. Fagundes, J. M. Prado, M. H. Toyama, M. A. A. Meireles, "Brazilian ginseng extraction via LPSE and SFE: global yields, extraction kinetics, chemical composition and antioxidant activity", J. Supercrit. Fluids, vol.54, no.1, pp.38-45, 2010. |
| [17] | M. A. A. Meireles, P. F. Leal, F. C. Alexandre, M. B. Kfouri, "Extraction process of active substances from brazilian ginseng", PI0900551-0A2, Brazil, 2009. |
| [18] | T. M. Takeuchi, M. L. Rubano, M. A. A. Meireles, "Characterization and functional properties of macela (Achyrocline satureioides) extracts obtained by supercritical fluid extraction using mixtures of CO2 plus ethanol", Food Bioproc. Technol., vol.3, no.6, pp.804-812, 2010. |
| [19] | R. N. Cavalcanti, H. J. J. Navarro-Díaz, D. T. Santos, M. A. Rostagno, M. A. A. Meireles, "Supercritical carbon dioxide extraction of polyphenols from pomegranate (Punica granatum L.) leaves: chemical composition, economic evaluation and chemometric approach", J. Food Research, vol.1, no.3, pp.282-294, 2012. |
| [20] | R. N. Cavalcanti, P. C. Veggi, M. A. A. Meireles, "Supercritical fluid extraction with a modifier of antioxidant compounds from jabuticaba (Myrciaria cauliflora) byproducts: economic viability", Procedia Food Sci., vol.1, pp.1672-1678, 2011. |
| [21] | P. C. Veggi, "Obtaining vegetable extracts by different extraction methods: experimental study and process simulation", Master Thesis, University of Campinas/ Brazil/ Department of Food Engineer, Campinas-SP/Brazil, 2009. |
| [22] | M. A. A. Meireles, P. T. V. Rosa, "Extraction and purification process of artemisin from solid mass of Artemisia annua using carbon dioxide", PI0903275-4A2, Brazil, 2009. |
| [23] | E. Rahimi, J. M. Prado, G. Zahedi, M. A. A. Meireles, "Chamomile extraction with supercritical carbon dioxide: mathematical modeling and optimization", J. Supercrit. Fluids, vol.56, no.1, pp.80-88, 2011. |
| [24] | C. L. C. Albuquerque, M. A. A. Meireles, "Defatting of annatto seeds using supercritical carbon dioxide as a pretreatment for the production of bixin: experimental, modeling and economic evaluation of the process", J. Supercrit. Fluids, vol.66, pp.86-95, 2012. |
| [25] | J. M. Prado, "Scale-up study of supercritical fluid extraction process in fixed bed", Doctoral in Food Engineering, Unicamp/Food Engineering, Campinas-Brazil, 2010. |
| [26] | G. L. Zabot, M. N. Moraes, M. A. A. Meireles, "Supercritical fluid extraction of bioactive compounds from botanic matrices: experimental data, process parameters and economical evaluation", Recent Patents Eng., vol.6, no.3, pp.182-206, 2012. |
| [27] | J. M. Prado, G. H. C. Prado, M. A. A. Meireles, "Scale-up study of supercritical fluid extraction process for clove and sugarcane residue", J. Supercrit. Fluids, vol.56, no.3, pp.231-237, 2011. |
| [28] | G. L. Zabot, M. N. Moraes, A. J. Petenate, M. A. A. Meireles, "Influence of the bed geometry on the kinetics of the extraction of clove bud oil with supercritical CO2", Journal of Supercritical Fluids, 2013, In press. |
| [29] | J. M. Prado, I. Dalmolin, N. D. D. Carareto, R. C. Basso, A. J. A. Meirelles, J. V. Oliveira, E. A. C. Batista, M. A. A. Meireles, "Supercritical fluid extraction of grape seed: process scale-up, extract chemical composition and economic evaluation", J. Food Eng., vol.109, no.2, pp.249-257, 2012. |
| [30] | D. T. Santos, "Extraction, micronization and stabilization of functional pigments: construction of multipurpose unit for pressurized fluid process development", Doctoral Thesis, University of Campinas/Brazil, Department of Food Engineering, Campinas-SP/Brazil, 2011. |
| [31] | D. T. Santos, M. A. A. Meireles, "Micronization and encapsulation of functional pigments using supercritical carbon dioxide", J. Food Process Eng., vol.36, no.1, pp.36-49, 2011. |
| [32] | D. T. Santos, J. Q. Albarelli, M. M. Beppu, M. A. A. Meireles, "Stabilization of anthocyanin extract from jabuticaba skins by encapsulation using supercritical CO2 as solvent", Food Research Int., vol.50, no.0, pp.617-624, 2012. |
| [33] | D. T. Santos, A. Martin, M. A. A. Meireles, M. J. Cocero, "Production of stabilized sub-micrometric particles of carotenoids using supercritical fluid extraction of emulsions", J. Supercrit. Fluids, vol.61, pp.167-174, 2012. |
| [34] | D. T. Santos, D. F. Barbosa, K. Broccolo, M. T. M. S. Gomes, R. Vardanega, M. A. A. Meireles, "Pressurized organic solvent extraction with on-line particle formation by supercritical anti solvent processes", Food Public Health, vol.2, no.6, pp.231-240, 2012. |
| [35] | M. T. M. S. Gomes, D. T. Santos, M. A. A. Meireles, "Trends in Particle Formation of Bioactive Compounds Using Supercritical Fluids and Nanoemulsions", Food Public Health, vol.2, pp.142-152, 2012. |
| [36] | D. T. Santos, D. F. Barbosa, R. Vardanega, J. Q. Albarelli, M. A. A. Meireles, "Experimental and simulation study on formulation of clove essential oil products using alternative surfactant", J. Coll. Sci. Biotech., vol.2, pp.112-122, 2013. |
| [37] | D. T. Santos, D. F. Barbosa, R. Vardanega, M. T. M. S. Gomes, M. A. A. Meireles, "Novel method to produce emulsions containing essential oils from saponin-rich pressurized aqueous plant extracts", J. Coll. Sci. Biotech., vol.2, pp.93-99, 2013. |
| [38] | L. M. Rodrigues, S. C. Alcázar-Alay, A. J. Petenate, M. A. A. Meireles, "Bixin extraction from defatted annatto seeds", Comptes Rendus Chimie, 2014, In press. |
| [39] | J. M. Prado, L. A. Follegatti-Romero, F. P. Cardenas-Toro, T. Forster-Carneiro, M. A. Rostagno, M. A. A. Meireles, 2012, Cellulose and sugarcane bagasse hydrolysis using subcritical water, Proc. 10th International Symposium on Supercritical Fluids, San Francisco, CA-USA. |
| [40] | L. A. Follegatti–Romero, J. M. Prado, T. F. Carneiro, F. P. Cardenas–Toro, M. A. Rostagno, M. A. A. Meireles, 2012, Semi–batch equipment for the hydrolysis of lignocellulosic raw materials, Proc. 10th International Symposium on Supercritical Fluids, San Francisco, CA-USA. |
| [41] | P. C. Veggi, D. T. Santos, M. A. A. Meireles, "Anthocyanin extraction from Jabuticaba (Myrciaria cauliflora) skins by different techniques: economic evaluation", Procedia Food Sci., vol.1, pp.1725-1731, 2011. |
| [42] | J. M. Prado, A. R. Assis, M. R. Marostica, M. A. A. Meireles, "Manufacturing cost of supercritical-extracted oils and carotenoids from amazonian plants", J. Food Proc. Eng., vol.33, no.2, pp.348-369, 2010. |
| [43] | B. Tan, "Appropriate spectrum vitamin E and new perspectives on desmethyl tocopherols and tocotrienols", Journal of American Nutraceutical Association, vol.8, pp.35-42, 2005. |
| [44] | V. Akshatha, P. Giridhar, G. A. Ravishankar, "Morphological diversity in Bixa orellana L. and variations in annatto pigment yield", Journal of Horticultural Science and Biotechnology, vol.86, pp.319-324, 2011. |
| [45] | D. T. Santos, M. A. A. Meireles, "Optimization of bioactive compounds extraction from jabuticaba (Myrciaria cauliflora) skins assisted by high pressure CO2", Innov. Food Sci. Emerg. Technol., vol.12, no.3, pp.398-406, 2011. |
| [46] | D. T. Santos, P. C. Veggi, M. A. A. Meireles, "Extraction of antioxidant compounds from jabuticaba (Myrciaria cauliflora) skins: yield, composition and economical evaluation", J. Food Eng., vol.101, no.1, pp.23-31, 2010. |
| [47] | D. T. Santos, C. L. C. Albuquerque, M. A. A. Meireles, "Antioxidant dye and pigment extraction using a homemade pressurized solvent extraction system", Procedia Food Sci., vol.1, pp.1581-1588, 2011. |
| [48] | D. T. Santos, J. Q. Albarelli, M. A. A. Meireles, "Simulation of an integrated sustainable production of extract from Brazilian ginseng roots with a cogeneration plant", Chem. Eng. Transactions, vol.29, pp.91-96, 2012. |
| [49] | R. Vardanega, D. T. Santos, I. C. N. Debien, M. A. A. Meireles, 2012, Pressurized liquid extraction of bioactive compounds from brazilian ginseng roots (Pfaffia glomerata), Proc. 16th world congress of food science and technology, Foz do Iguaçu-PR/Brazil. |
| [50] | A. M. Farías-Campomanes, M. A. Rostagno, M. A. A. Meireles, "Production of polyphenol extracts from grape bagasse using supercritical fluids: yield, extract composition and economic evaluation", J. Supercrit. Fluids, vol.77, pp.70-78, 2013. |
| [51] | T. Hatami, M. A. A. Meireles, G. Zahedi, "Mathematical modeling and genetic algorithm optimization of clove oil extraction with supercritical carbon dioxide", J. Supercrit. Fluids, vol.51, no.3, pp.331-338, 2010. |
| [52] | T. Hatami, R. N. Cavalcanti, T. M. Takeuchi, M. A. A. Meireles, "Supercritical fluid extraction of bioactive compounds from macela (Achyrocline satureioides) flowers: kinetic, experiments and modeling", J. Supercrit. Fluids, vol.65, pp.71-77, 2012. |
| [53] | A. R. Assis, "Assembly, test and validation of a supercritical extraction unit with recycle and continuous operation", Doctoral Thesis, University of Campinas/Brazil, Department of Food Engineering, Campinas-SP/Brazil, 2010. |
| [54] | T. M. Takeuchi, "Supercritical extraction of macela, clove and vetiver: technological and economical aspects", Doctoral Thesis, University of Campinas/Brazil, Department of Food Engineering, Campinas-SP/Brazil, 2009. |
| [55] | D. T. Santos, R. N. Cavalcanti, M. A. Rostagno, C. L. Queiroga, M. N. Eberlin, M. A. A. Meireles, "Extraction of polyphenols and anthocyanins from the jambul (Syzygium cumini) fruit peels", Food Public Health, vol.3, no.1, pp.12-20, 2013. |
| [56] | P. C. Veggi, D. T. Santos, A. S. Fabiano-Tixier, C. Bourvellec, M. A. A. Meireles, F. Chemat, "Ultrasound-assisted extraction of polyphenols from jatoba (Hymenaea courbaril L. var stilbocarpa) bark", Food Public Health, vol.3, no.3, pp.119-129, 2013. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML