-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(1): 10-14
doi:10.5923/j.fph.20140401.02
Effect of Processing on the Proximate and Mineral Composition of Archachatina marginata and Achatina achatina
Friday E. Uboh1, Ima O. Williams1, Nessie C. Essien2
1Department of Biochemistry, Faculty of Basic Medical Sciences, University of Calabar, Calabar, Nigeria
2Department of Nursing Science, Faculty of Allied Medical Sciences, University of Calabar, Calabar, Nigeria
Correspondence to: Friday E. Uboh, Department of Biochemistry, Faculty of Basic Medical Sciences, University of Calabar, Calabar, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Proximate and mineral nutrients composition of boiled and roasted samples of two African giant land snails Archachatina marginata and Achatina achatina were assessed in this study. The study aimed at investigating the effect of two cultural methods of preparing snails for consumption in Nigeria (boiling and roasting) on the nutritional quality of the snail meat. Proximate analysis was carried out following the methods of the Association of Official Analytical Chemists, while mineral nutrients were analyzed using atomic absorption spectrometry. The results showed that roasting affected the nutrient composition of the two snail species more than boiling. In A. marginata, only the carbohydrate content was increased by boiling (unprocessed 22.53 ± 1.08%, boiled 34.68±2.34%), whereas roasting of the same sample led to increased carbohydrate (32.62 ± 2.20%), protein (66.85± 3.01%), and fat (3.02 ± 0.18%) contents (unprocessed 22.53 ± 1.08%, 63.46 ± 2.56%, and 2.40 ± 0.02%, respectively). Similarly, in A. achatina only the carbohydrate content was increased by boiling (33.82± 2.16%), while roasting increased both the carbohydrate (31.28 ± 2.00%) and protein (66.58 ± 2.04%) contents (unprocessed 24.38 ± 1.29 and 63.46 ± 2.56%, respectively). Boiling increased the concentration of all the mineral elements assayed in A. marginata and A. achatina except Na, while roasting increased the concentration of all the mineral elements in the two snail species. Thus, to achieve higher levels of nutrients in snails, it may be advisable to roast rather than boil the flesh. Also, the two snail species should be cultivated and consumed as a good, cheapand alternative source of protein as there is no difference in their nutrient composition.
Keywords: Snails, Processing Methods, Nutrients, Toxicants
Cite this paper: Friday E. Uboh, Ima O. Williams, Nessie C. Essien, Effect of Processing on the Proximate and Mineral Composition of Archachatina marginata and Achatina achatina, Food and Public Health, Vol. 4 No. 1, 2014, pp. 10-14. doi: 10.5923/j.fph.20140401.02.
Article Outline
1. Introduction
- Several groups of mollusks are known to be available in nature, with snails constituting the largest group[1]). The snails are also made up of several species. Among the different species commonly found in the Southern part of Nigeria include the two species of African giant land snails, the black snail (Archachatina marginata) and the white snail (Archachatina achatina). These snails are commonly found in those areas where the tropical weather and vegetation conditions are most favourable for their proliferation. Generally, these species of snails are reported to be found in the infringing forest of the derived Guinea Savannah in West Africa[2]. The major distinctive feature between the two snail species is the colour of the body (i.e., the edible part).While black snail (A. marginata) has a brownish body colour, the white snail (A. achatina) is whitish in body colour (Figures 1 and 2). The snails are found to thrive well in wet and damp soft soils either wildly on their own, or in a confinement for domestic consumption or commercial purposes[2]. The indiscriminate hunting and deforestation destroys the habitat and reduces the abundance of giant land snails. Rearing of the giant land snails as a domestic animal would therefore help in some measure, to ensure the survival of the species and satisfy the increasing demand for protein.The major sources of meat protein for most of the African population, including Nigerians, come mainly from livestock in the form of poultry, beef, mutton and pork. These sources have been decreasing by persistent drought, diseases, high cost of feed, primitive animal husbandry techniques and low productivity of local animal breeds. Particularly, the increasing growth of human populations[3] together with low per capita income of a vast majority of the population and the rising standard of living has also placed great pressure on the existing sources of animal protein. Meat from different species of snail is reported to be rich in protein, low in fats and a source of iron[4],[5],[6],[7]. Studies on the nutritional value of snail have therefore reported that snail is high in protein quality but low in fat contents hence an alternative food for people with high protein quality low fat diet requirements[8],[9],[10].The land snails, particularly the A. marginata, are alternative and non-conventional animal protein source in Nigeria and some other parts of Africa. In some of the Nigerian societies, the snail meat (also known as “Congo meat”) is becoming a highly relished delicacy. Although the nutritional composition of a variety of foods has been known for many years, relatively little information has been collected on the nutritional composition of edible snail. Hence snails are therefore supposed to be highly recommended as an additional source of animal protein globally. However, there is a strong cultural discrimination in consumption of some species of snails in different parts of the world. For instance, some tribes in the southern part of Nigeria prefer the black snail (A. marginata) and discriminate the white snail (A. achatina), although there is no documented scientific justification for this discrimination. Snails are processed by different methods for consumption. While some people eat snails in roasted form, others either boil them in the course of cooking or fry them for consumption. There is paucity of documented information on whether these processing methods have any effect on the proximate and mineral elements compositions of these processed snail meat. However, Onyeike and Oguike[11], and Ojiako et al.[12] reported that different processing methods, including boiling and roasting, influence the proximate, mineral and toxicant compositions of foods. Hence, this study aimed at assessing whether the proximate and mineral elements composition of black snail (A. marginata) differ from that of the white snail (A. achatina), as well as determining whether boiling and roasting methods of food processing have any significant effect on the proximate and mineral elements compositions of the two snail species.
 | Figure 1. dorsal view of crawling (a) black snail (A. marginata) and (b) white snail (A. achatina) |
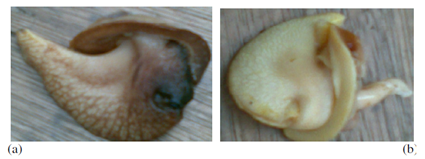 | Figure 2. Edible part (i.e., the body) of (a) black snail (A. marginata) and (b) white snail (A. achatina) |
2. Materials and Methods
2.1. Collection and Preparation of Samples
- A total of 120 snails each, of both species of snails were sampled in this study. A. achatina were randomly picked within the University campus, while A. marginata were purchased from a local market in Odukpani, Cross River State, Nigeria. The shells were carefully removed so that the edible parts (i.e., the body) shown in Figure 2 could be extracted and processed for analysis. The snails in each of the species were divided into two groups (60 each), one for boiling and the other for roasting. The snails in the group meant for boiling were boiled for 30 minutes and designated “boiled sample”, while the snails in the group meant for roasting were roasted in an oven at 80°C for 45 minutes and designated “roasted sample”. The boiled and roasted samples were then further processed and analysed for the proximate and mineral elements compositions.
2.2. Proximate Composition Analysis
- Ash and crude fibre compositions of the boiled and roasted snail samples were determined by the method of the Association of Official Analytical Chemists (AOAC)[13]. Nitrogen was determined by the Micro-Kjeldahl method as described by Pearson[14] and the percentage nitrogen was converted into crude protein by multiplying by 6.25. Fat content was determined by the method of Bligh and Dyer[15]. Carbohydrate was estimated by the difference between the sum of the values of the previous nutritional components (protein, moisture, fibre, fat and ash) and 100% (accepted overall value of nutritional components).
2.3. Determination of Mineral Elements
- The mineral compositions of the boiled and roasted samples were analyzed from solutions obtained by first dry-ashing the samples at 550 °C and dissolving the ash in standard flasks with distilled, de-ionized water containing a few drops of concentrated hydrochloric acid. Phosphorus was determined colorimetrically from the prepared sample using spectronic-20 colorimeter (Gallenkamp, UK) as described by Pearson[14] with KH2PO4 as a standard. Sodium and potassium were analysed by means of flame photometer (Model 405, Corning, UK), using NaCl and KCl to prepare the standards. Calcium, magnesium, iron, zinc, cadmium, chromium, lead and mercury were analysed by means of atomic absorption spectrophotometry (Models SP 9, Pye Unicam, UK).
2.4. Quality Assurance
- To ensure the reliability of the results, appropriate quality assurance procedures and precautions were observed for all the parameters analysed in the laboratory. All the samples were carefully handled to prevent them from being contaminated. Also, the glassware was properly cleaned and all the reagents used in this study were of analytical grade. Double distilled deionized water was used throughout the study, and reagents blank determinations were used to correct the instrument readings.
2.5. Statistical Analysis
- The proximate and mineral elements compositions obtained for A. achatina were compared with the respective composition obtained for A. marginata. Also, the proximate and mineral elements compositions recorded for boiled samples of both species were compared with the respective composition recorded for the roasted samples. The results obtained were analyzed using Student’s t test with statistical significance considered for P < 0.05.
3. Results
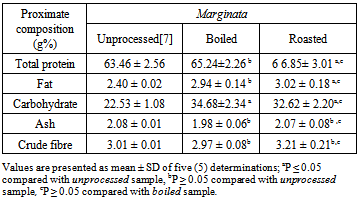
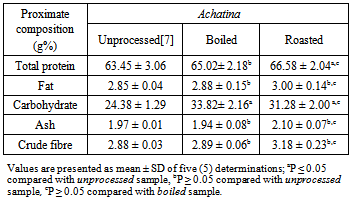
3.1. Proximate Composition
- The results of proximate analysis are presented in Tables 1 and 2. The carbohydrate content of the boiled (34.68±2.34%) A. marginata was significantly (p<0.05) higher than the unprocessed (22.53 ± 1.08%) sample, while the protein, fat, ash and crude fibre contents were not affected by boiling. In contrast, roasted A. marginata was higher (p<0.05) in carbohydrate (32.62 ± 2.20%), total protein (6 6.85± 3.01%), and fat (3.02 ± 0.18%) contents than the unprocessed form; only ash and crude fibre were not affected by roasting. However, there was no difference (p<0.05) in the proximate composition of boiled and roasted A. marginata.
|
|
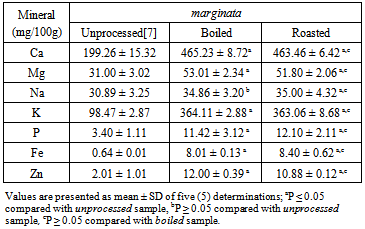
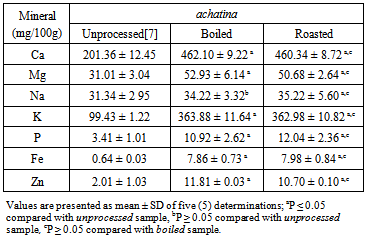
3.2. Composition of Mineral Elements
- The results of the mineral composition of the boiled and roasted samples of the two snail species are shown in Tables 3 and 4. There were higher (p<0.05) levels of Ca, Mg, K, P, Fe, and Zn in the boiled than unprocessed A. marginata. However, Na content of the boiled and unprocessed (34.86 ± 3.20% and 30.89±3.25%, respectively) samples were similar (p>0.05). Roasted A. marginata showed higher levels of all the minerals than the unprocessed form. There were no significant differences in the mineral levels of the boiled and roasted A. marginata meat.
|
|
4. Discussion
- Various processing methods have been reported to influence the nutrient contents of mostfoods[11],[12],[16], [17]. In this study, roasting was observed to have a significant influence on proximate and mineral contents of snail samples more than boiling. The proximate and mineral compositions of Archachatina marginata samples were not significantly different from the contents recorded for the Achatina achatina samples. The results of this study suggest that the effect of processing methods on the nutrients composition of foods may be dependent on the nature or type of the food sample, as well as other factors including the processing time and temperature. Roasting of meat takes longer time and higher temperature. These factors may be responsible for the greater increase in the proximate and mineral constituents of the snail meat samples recorded with roasting than with boiling.Snail meat is reported to be a high quality food that is rich in protein, low in fat, and a source of many vital minerals required for normal tissue development and maintenance[6], [7],[8],[9],[10],[19],[20][21]. The results of the present study support these reports. The present study also support the findings that the protein content of snail is comparable with the protein content of donkey meat[6], and higher than the protein content of beef[18]. Furthermore, the present study reveals that the protein content of both species of snail, A. achatina and A. marginata, are highly comparable. However, the results here, like that of our previous study on the protein content of the two species of snail[7], disagree with the findings by Fagbuaro et al.[20], who observed that the protein composition of snails differ among the four species of African giant land snails; (A. marginata (ovum) Pfeiffer, A. marginata (saturalis) Philippi, A. achatina and Limicolaria spp. The results of their studies on the proximate and mineral compositions of the meat of the four species of African giant land snails showed that on the basis of wet weight, the crude protein composition varied from 18.66%±0.57% in Limicolaria spp. to 20.56%±0.05% in A. marginata (ovum) Pfeiffer. Also, moisture content of 76.56%±0.04% in A. marginata (ovum) Pfeiffer and 78.68%±0.68% in Limicolaria spp were reported. The ash content of 1.34%±0.02% in A. achatina and 1.44%±0.01% in A. marginata (ovum) Pfeiffer were also reported by the authors [20]. The results of this study also agree with the results reported by Çağıltay et al.[10], Olgunoğlu and Olgunoğlu[22], and Özden and Erkan[23] for garden snail and some sea food species, including mollusks. Garden snail meat has similar levels of protein, amino acids, vitamins, minerals and fatty acids with other seafoods. It is therefore an important food source to supply daily nutritional needs.The results of this study, in agreement with our previous work[7] and part of the report of Fagbuaro et al.[20], indicate that the concentration of calcium, magnesium, sodium, potassium, phosphorus, iron and zinc were consistently high in different species of snail. Also, the mineral composition of the two species of snail compare favourably with the mineral contents of some lean domestic livestock meats[18]. These reports give an indication that snails may be used to complement the required macro- and micro- elements needed for proper growth and development in human beings and other domesticated animals. Minerals are known to play important roles in the maintenance of various biochemical activities in the biological systems. In particular, calcium was recorded in high concentration in the two species of snail. Calcium is known to play an important role in blood clotting and bone development in mammals, including human beings. Pearson and Gillet[24] reported that calcium is the most abundant mineral element in the animal body and an important constituent of the skeleton and teeth, where around 99% of the total calcium in the body is found. Calcium is also essential for the activity of a number of enzyme systems, including those necessary for the transmission of nerve impulses. Magnesium is a key element in cellular biochemistry and function. Magnesium is closely associated with calcium and phosphorus and about 70% of the total Mg is found in the skeleton. Magnesium is an enzyme activator, for example in systems with thiamine pyrophosphate as a co-factor and oxidative phosphorylation is reduced in Mg deficiency. It is an essential activator of phosphate transferase, activates pyruvate carboxylase, pyruvate oxidase and the reactions of the tricarboxylic acid cycle. Potassium is also known to play an important role in osmotic regulation of the body fluids and in acid-base balance in the animal. It also participates in nerve and muscle excitability as well as in carbohydrate metabolism[18].
5. Conclusions
- The present study confirms that the two species of snail (A. achatina and A. marginata) are good sources of protein and mineral nutrients which can be obtained at minimal cost, considering the high cost and associated high fat content risk of beef, poultry and other higher meat proteins. Hence, consumption of the two species of giant land snails should be encouraged for both the young and old, as an alternative source of essential nutrients at a lower cost.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML