-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(6): 346-351
doi:10.5923/j.fph.20130306.13
Effect of Conventional and Oven Drying on the Physicochemical Properties of Two Tomato Cultivars Fruit Powder
Abdel Moneim E. Sulieman1, Sara A. Abdalla2, Zakaria A. Salih2
1Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia
2Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
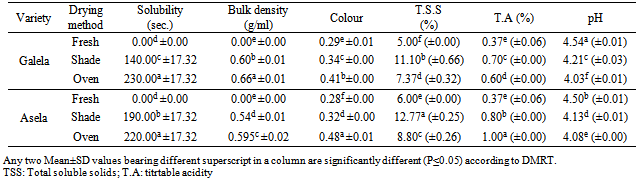
The aim of the present study was to investigate the effect of conventional and oven drying on the physicochemical properties of Aseela and Galeela tomato cultivars fruit powder. Most of the studied parameters were significantly affected by the employed drying processes. The results showed that the bulk density of the fresh tomato from the two cultivars was (0), whereas in the shade-dried tomato, the bulk density was 0.60 % and 0.54% in Galela tomato powder (GTP) Asela tomato powder (ATP), respectively. However, in the oven dried method, bulk density was 0.66 and 0.56 in GTP and ATP, respectively. It was found that the shaded dried tomato of the variety Asela took longer time to dissolve (190 sec), followed by (140 sec) for the variety Galela, while in oven-dried tomato, the cultivar Galela took longer time to dissolve (230sec), followed by (220 sec) for the cultivar Asela. The total soluble solids (T.S.S.) of fresh tomato (FT), shade dried tomato and oven dried tomato prepared from Galela tomato cultivar was 5%, 11.10% and 7.37%, respectively. Whereas was in tomato powder prepared from Asela cultivar, the T.S.S. of FT, shade dried and oven dried was 6 %, 12.77% and 8.80%, respectively. soluble solids from 4.79 to 6.02%, depending on the cultivar. The results of titrable acidity was found to be 0.37, 0.70 and 0.60% in FT, shade dried tomato and oven dried tomato powder, respectively for the variety Galela, and 0.37, 0.80, 1.0 % for the variety Asela. The obtained results clearly indicate that the investigated oven dry tomatoes compared to the shade dry cultivars have a satisfying quality and nutritional value.
Keywords: Shade Drying, Wettability, Sinkability, Solubility, Bulk Density
Cite this paper: Abdel Moneim E. Sulieman, Sara A. Abdalla, Zakaria A. Salih, Effect of Conventional and Oven Drying on the Physicochemical Properties of Two Tomato Cultivars Fruit Powder, Food and Public Health, Vol. 3 No. 6, 2013, pp. 346-351. doi: 10.5923/j.fph.20130306.13.
Article Outline
1. Introduction
- Vegetables and fruits can be processed and preserved by drying. Drying preserves food because the microorganisms that spoil food need water to grow. Drying also concentrates a food's nutrients and preserves them for times when fresh food is not available. Improved technologies, such as solar dryers, retain higher quantities of vitamins in food than can be retained using the traditional method of sun drying[1].Drying is one of the oldest methods of food preservation. Drying preserves foods by removing enough moisture from food to prevent decay and spoilage. Water content of properly dried food varies from 5 to 25 percent depending on the food. Successful drying depends on: enough heat to draw out moisture, without cooking the food, dry air to absorb the released moisture, and adequate air circulation to carry off the moisture[2].Shade drying requires full air circulation. It should not be undertaken inside conventional buildings but in an open-sided shed purposely built for shade drying. Most foods to be dried are sliced (e.g. peppers, okra, onions, tomatoes, eggplants, yams, sweet potatoes and carrots), as sliced food generally dries faster. Drying ovens are designed to remove moisture. Typical applications are pre-treating and painting. Such ovens are also sometimes known as kilns, though they do not reach the same high temperatures as are used in ceramic kilns[3].Tomatoes (Lycopersicon esculentun Mill) are classified according to their use as fresh consumption and processing. Both categories of tomato should be sound, firm, mature, having deep uniform red color, free from cracks and green shoulders and poisonous material and eventually should be nationally valuable. Furthermore, tomato for table use should be juicy, characterized by high total soluble solids, fairly low acidity, high non-fibrous pulp content[4].Tomato powders are often used as an ingredient in the foods such as sauces and soups. Several food technology studies have been carried out to optimise the processing and storage of the tomato products by preventing the heat and oxidative damage on the antioxidants[5]. The aims of this study were to study the effect of conventional and oven drying on the physicochemical properties of two tomato cultivars fruit powder.
2. Materials and Methods
2.1. Preparation of the Raw Material
- Two cultivars of tomato (Galela and Asela) were collected in polyethelene bags (plastic containers) from green houses of the Date Palm Technology Co, Shambat, Khartoum, during May 2011. The tomato fruits were sorted from injured and deteriorated fruits, washed under running tap water and weighed. The cleaned fruits were cut into small slices (one cm in length) using sharp sterilized stainless steel knives, and then were placed into trays pending dehydration process.
2.2. Dehydration of Tomato Slices
- The tomato slices were spread on perforated stainless steel trays (45cm wide, 75cm long and about 7cm height (for the under shade samples) and (34 cm wide, 50 cm long and about 5 cm height (for the drier samples). One and half (wet weight) of tomato slices were loaded on a perforated stainless steel trays and left to dry under shade with the aid of fans for 4 days. The rest of the samples were put into oven drier under different temperature (90, 80, 70, 65 and 60ºC ) for two days.
2.3. Preparation of Tomato Powder
- The dried tomato slices were collected, reweighed and ground using alcohol cleaned household grinder and stored in deep freezer into sealed plastic bags prior to further analysis.
2.4. Physicochemical Properties
2.4.1. Evaluation of the Reconstitution Characteristics for the Tomato Powder
- Reconstitution characteristics which included Wettability and sinkability, solubility, bulk density, sorption isotherm rate of tomato powder were carried out for fresh tomato and tomato powder samples prepared from the two tomato cultivars.
2.4.2. Wettability and Sinkability
- Wettability and sinkability of powders are difficult to separate and they were done in one test. The test was started by spreading five grams of air dried tomato powder on the surface of a filter paper (No. 5), held tightly between the gaps of two small food cans ( just enough to pull) where the cans were opened at both ends. The assembly of the two cans and the filter paper were mounted on a glass beaker (500 ml) containing 500 ml distilled water that; paring in mind; the height of the surface of the beaker and end of the apparatus is 3 inches. They were left to immerse and then; the time for the powder to be wetted was recorded, on the other hand time taken by the powder to sink down was also recorded[6].
2.4.3. Solubility Rate
- The solubility was determined according to Neff and Morries[6] by adding 5grams of the samples to 150 ml of distilled water at room temperature (35 + 0.5ºC) in a 400ml beaker, the mixture was immediately stirred using a magnetic stirrer at 1000 rpm to assured systematic stirring, meanwhile the time for complete solubility was counted.
2.4.4. Bulk Density
- The bulk density is expressed in grams/ml as described by Neff and Morris[6]. Twenty grams sample of the developed powder were weighed and then transferred to a graduate 100 ml measuring cylinder and mounted on screen vibrator, shaken for five minutes. The bulk density was obtained by measuring the volume occupied in the cylinder.
2.4.5. Color Intensity (Optical Density)
- Two grams of developed powder of tomato produced were weighed (in triplicate). The samples were transferred to 250 ml Beakers, then 100 ml of 50% ethanol were added and the beakers were covered with paraffin film, left for overnight at room temperature with occasional shaking. The solutions were filtered through Buchner funnel using Whatman filter paper (No.1). The optical density was measured by the spectrophotometer (Analyzer-9) at 420nm using 0.22cm diameter tube[7].
2.4.6. Determination of Total Soluble Solids (TSS) Content and Titratable Acidity (TA)
- Total soluble solids (TSS) were determined for each sample according to AOAC[8] method using an Atago DR-A1digital refractometer (Atago Co. Ld., Japan) at 25°C and expressed as percentage. Titratable acidity (TA) was obtained by titrating 5 ml of tomato powder with 0.1 N NaOH up to pH 8.1. The result was expressed as grams of citric acid per 100 g of fresh tomato weight.
2.4.7. Determination of pH
- The pH was measured using Hanna pH meter at ambient temperature 35 ±5. Five grams of the raw material and equivalent weight in grams of the soluble matter in the raw material of tomato dried powder considering moisture content differences, The samples were dissolved in 50 ml distilled water and filter through Whatman filter paper No.1. Then the pH meter calibrated within the range of 4 – 9 pH and the pH of the samples was measured in triplicates.
2.4.8. Sorption Isotherm
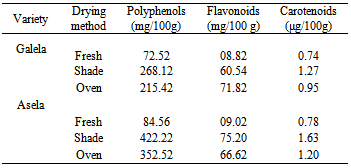
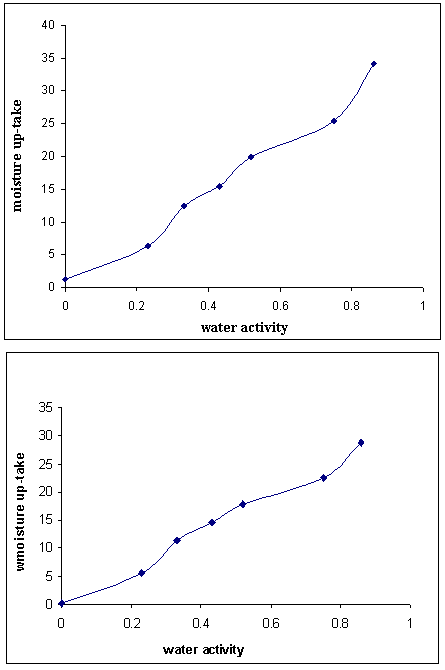
- The method was described by Wink[9] (1964) as follows; Ten grams sample were weighted accurately in petri dishes 7cm in diameter and placed in closed desiccators in which the relative humidity was controlled by saturated salt solution. The saturated salt solution used were; sulphuric acid (0%R.H.); potassium acetate (23% R.H.) magnesium chloride (33%R.H.); potassium carbonate (43% R.H.); magnesium nitrate (52%R.H.); sodium nitrate (75%R.H.) and potassium chloride ( 86%R.H.) the temperature was kept at 30°C ± 2. The initial moisture content of the samples was determined periodically, at an intervals of 24 hours until constant weights in three successive weightings. The equilibrium moisture content were calculated and then plotted in the Y- axis against the water activity in the X-axis .(water activity was drawn from the values of the equilibrium relative humidity divided by 100).Preparation of methanolic extract For preparation of methanolic extract, dried tomato slices (10 g) were stirred with 100 mL MeOH at 30°C for overnight. The extract was filtered through Whatman no. 1 filter paper for removal of seed particles. The residue was re-extracted with 60 mL methanol. The obtained extracts were filtered again and concentrated under vacuum at 40°C. These methanolic extracts were used for phenolic and antioxidant analyses.Determination of total phenolic and flavonoid contentsThe total phenolic compounds were measured using Folin-Ciocalteu method according to Elfalleh et al.,[10]. In this method, from each sample, 0.5 mL of methanolic extract solution was added to 0.5 mL of Folin- Ciocalteu reagent (Prolabo, Paris France), followed by 4 mL of 1M sodium carbonate. Then, the test tubes were incubated at 45°C for 5 min and cooled in cold water. Absorbance was measured at 765 nm, using a spectrophotometer (Shimadzu, Kyoto, Japan). The results were compared to a gallic acid calibration curve, and the total phenolic compounds were determined as mg gallic acid equivalents per 100g dry weight basis (GAE mg/ 100g DW). Total flavonoids were also measured spectrophotometrically according Elfalleh et al.,[10]. This method based on the formation of a complex flavonoid – aluminium, having the maximum absorbance at 430 nm. Rutin was used to make a calibration curve. One mL of methanolic extract was mixed with 1 mL of 2% AlCl3 methanolic solution. After incubation at room temperature for 15 min, the absorbance of the reaction mixture was measured at 430 nm using a a spectrophotometer. The flavonoids content was expressed as mg rutin equivalents per 100 g dry weight basis (mg RE/100 g DW).Determination of total carotenoid contentsThe quantification of carotenoids as xanthophylls and carotenes entail with the determination of chlorophyll (Chl) Chla and Chlb by UV-VIS spectroscopy. Chlorophyll and carotenoids were extracted from tomato fruit using a method modified by Gitelson et al.[11] Briefly, samples were put into a pre-chilled tube, and ground for 3 min in 1 mL extraction buffer (80% acetone: Tris-HCl[1%, w/v]). After the pigments were completely extracted by the buffer, an additional 1 mL extraction buffer was used to wash the pestle. All extraction solutions were combined and debris was removed by centrifugation. A volume of 1 mL of the supernatant was diluted to 3 mL final solution. The light absorbance of the final solution was measured at 663, 647 and 470 nm. The concentrations of carotenoids and chlorophyll were calculated as described by Lichtenthaler [12]. All experiments were done in triplicate and the carotenoids contents were converted to mg per kg of fresh weight.
3. Results and Discussion
- The physicochemical properties of the shade-dried tomato powder and oven-dried tomato are shown in Table (1) and Fig. 1-2.As shown in Table (1), the bulk density of the fresh tomato from the two cultivars was (0), whereas in the shade-dried tomato, the bulk density was 0.60 % and 0.54% in Galela tomato powder (GTP) Asela tomato powder (ATP), respectively. However, in the oven dried method, bulk density was 0.66 and 0.56 in GTP and ATP, respectively. The bulk density values were greater than those of spray dried as reported by Asanathia and Konstantinos[13].
|
 | Figure 1. (A)Water Sorption Isotherm for Galela drier dried tomato at35±5.0°C (B): Water Sorption Isotherm for Galela shade dried tomato at35±5.0°C |
 | Figure 2. (A): Water Sorption Isotherm for Asela dried tomato sample at35±5.0°C B: Water Sorption Isotherm for Asela shade dried tomato sample at 35±5.0°C |
|
4. Conclusions
- In order to protect physicochemical properties and nutritional quality of tomato during dehydration process, investigation was carried out using two drying methods; shade drying and oven drying to dry samples of two tomato local tomato cultivars, Galela and Asela. Based on the results, drying of tomato has resulted in producing tomato powder with satisfactory quality and nutritive value. Drying process using the two techniques, shade and oven have an obvious impact on the physico-chemical properties of dried tomato.Both shade drying and oven drying can reduce the drying time and successfully would be used to produce good quality dried tomatoes. However, most of the physicochemical properties were not much affected by the type of tomato cultivar. Future studies to are needed to ensure safety, stability, optimum storage conditions, and suitable packaging requirements.
ACKNOWLEDGEMENTS
- The authors express their sincere gratitude for the staff members of Dehydration unit of the Food Research Centre, Shambat who presented valuable assistance in execution of the experimental work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML