-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(6): 304-308
doi:10.5923/j.fph.20130306.06
Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Phenolic Compounds of Carob (Ceratonia Siliqua L.)
M. Kamal E. Youssef1, Moshera M. El-Manfaloty2, Hend M. Ali2
1Food Science & Technology Department, Faculty of Agric., Assiut University, Assiut, A. R. Egypt
2Home Economic Department, Nutrition and Food Science, Faculty of Specific Education, Assiut University, Assiut, A. R. Egypt
Correspondence to: Hend M. Ali, Home Economic Department, Nutrition and Food Science, Faculty of Specific Education, Assiut University, Assiut, A. R. Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The Carob is the fruit of an evergreen (CeratoniaSiliqua L.) cultivated in the Mediterranean area. The pulp represents 90% of the fruit. It has a high content of sugars and tannins and low contents of protein and fat. Carob powder or syrup is used as an ingredient in cakes and cookies and chocolate substitute. Carob powder contained high levels of carbohydrates (75.92%), (6.34% protein) and low level of fat (1.99%). The crude fiber content recorded 7.30%. Carob powder was rich source of Fe, Ca, Na, K, P and S as well as E, D, C, Niacin, B6 and folic acid. Carob powder consisted of 11 phenolic compound. Pyrogallol, catechol, chlorogenic and protocatechuic recorded the highest values, while coumarin, cinnamic, ferulic, gallic acid and vanillic recorded the least values of the phenolic compounds. Carob powder oil consisted of 17 fatty acids, but consisted mainly of four fatty acids namely: oleic, linolic, palmitic and stearic acids recording 40.45%, 23.19%, 11.01% and 3.08%, respectively. Carob powder is acclaimed ingredient with a marked nutritional value due to its high dietary fiber and phenol compounds. The soluble fibers exert a preventative role against heart disease and lowering serum cholesterol.
Keywords: Carob Powder, Chemical Composition, Minerals, Vitamins, Phenolic Compounds, Fatty Acid Composition
Cite this paper: M. Kamal E. Youssef, Moshera M. El-Manfaloty, Hend M. Ali, Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Phenolic Compounds of Carob (Ceratonia Siliqua L.), Food and Public Health, Vol. 3 No. 6, 2013, pp. 304-308. doi: 10.5923/j.fph.20130306.06.
Article Outline
1. Introduction
- The Carob is the fruit of an evergreen (Ceratonia Siliqua) cultivated in the Mediterranean area. The seeds of the carob are utilized in food industry for their gum content. However, the use of the whole fruit in human consumption is rather limited, due to a high level of tannins causing astringency[1]. The fruit pod (containing sweet pulps) which is giving, after removal of the seeds and the carob powder[2] often used as a chocolate or cacoa substitute[3]. Carob fruits are used in food industry as a source of many products such as gum, sugar and alcohol[4],[5]. Recently investigators isolated and identified the major polyphenols in carob fibers[6, 7, 8] studied the variation and the composition of phenolic compounds of carob pods grown in different regions of Morocco. The chemical composition of carob was studied by[9]. The two principal components of the carob fruit are the pulp and seed. The seeds represent 10% of the weight of the fruit and the pulp represent the other 90% of the fruit, and its composition depends on the variety, climate and growing techniques[10, 11, 12 and 13].Carob is typically dried or roasted, and is mildy sweet. In powdered, chip, or syrup from it is used as an ingredient in cakes and cookies, and is used as a substitute for chocolate. Crushed pods may be used to make a beverage; compote, liqueur, and syrup are made from carob in Turkey, Malta, Portugal, Spain and Sicily[14]. Several studies suggest that carob may add in treating diarrhea in infants[15]. Carob powder is a natural sweetener with flour and appearance similar to chocolate; therefore it is often used as cocoa substitute. The advantage of using carob as a chocolate resides in that carob is an ingredient free from caffeine and theobromine[16]. Carob germ flour is used as dietetic human food[3], or as a potential ingredient in cereal – derived foods for celiac people[17].Furthermore, the protein content of carob germ flour seeds is higher than those observed for other beans such as faba bean, pea and soy beans[18]. Carob pulp is high (48 – 56%) in total sugar content that include many sucrose, glucose, fructose and maltose. In addition it contains about 18% cellulose and hemi cellulose. Ripe carob pods contain a large amount of condensed tannins (16 – 20%) on dry weight basis[19].Tradionally important main products of carob are pods, seed gums and derived products like carob bean flour, pekmez (concentrated carob syrup / molasses), health foods (as a chocolate substitute), carob syrup and medicines such as laxatives and diuretics[20, 21, 22, 23, 24, 25 and 26].The present investigation was carried out in an attempt to clarify the proximate chemical composition, the nutritional status, as well as, the fatty acid composition and the phenolic compounds of carob powder.
2. Materials and Methods
2.1. Materials
- 5 Kg. of carob powder were procured from Aswan Governorate in November 2012 where carob is cultivated. The seeds were removed and the carob was ground to particles of 1 ≤ mm. samples were stored at 18oC and analyzed within 2 months.
2.2. Methods
2.2.1. Determination of Gross Chemical Composition
- Moisture, crude protein, crude oil, crude fiber and ash contents were determined according to the procedures described in the AOAC[27]. The total carbohydrates were calculated by difference according to[28]. Caloric value was calculated according to[29].
2.2.2. Determination of Minerals Contents
- The samples were wet acid-digested using a nitric acid and perchloric acid mixture (HNO3; HCSO4; 2 : 1 v / v). The amounts of iron, zinc, copper and manganese in the digested sample were determined using a GBC Atomic Absorption 906 A, as described in[30]. Sodium and potassium were determined by a flame photometer 410, calcium was determined by titration with version 0.0156 N according to[31]. Phosphorus, sulphur and selenium were determined according to the methods described by[30].
2.2.3. Determination of Vitamins Contents
- Vitamin C was determined according to[32]. On the other vitamins E, D and A were determined according to the methods described by[33].
2.2.4. Determination of Vitamin B-complex
- A new reversed-phase chromatographic method was described for the separation and quantification of thiamine, folic acid, B12, pyridoxine, nicotinic acid and riboflavin applying HPLC technique as outlined by[34].
2.2.5. HPLC-separation and Identification of Phenolic Compounds
- Phenolic compounds were determined by HPLC according to the method of[35]. As follow: 5 g of sample were mixed with methanol and centrifuged at 10,000 rpm for 10 min and the supernatant was filtered through a 0.2 µm Millipore membrane filter then 1-3 ml was collected in a vial for injection into HPLC Hewllet Packared (series 1050) equipped with autosampliing injection, solvent degasser, ultraviolet (UV) detector set at 280 nm and quarter HP pump (series 1050). The column temperature was maintained at 35℃. Gradient separation was carried out with methanol and acetonitrile as a mobile phase at flow rate of 1 ml/min. phenolic acid standard from sigma Co. were dissolved in a mobile phase and injected into HPLC. Retention time and peak area were used to calculation of phenolic compounds concentration by the data analysis of Hewllet Packared software.
2.2.5.1. Preparation of Methyl Ester of Fatty Acids
- The methyl esters of fatty acids were prepared from aliquots of total lipids using 5 ml 3% H2SO4 in absolute methanol and 2 ml benzene as mentioned by[36].
2.2.5.2. GLC-of Methyl Ester of Fatty Acids
- The methyl esters of fatty acids were separated using a PYE Unicam Pro-GC gas liquid chromatography with a dual flame ionization carried out on (1.5m×4mm) SP-2310 column, packed with 55% cyanopropyl phenyl silicone dimensions. Column temperature: At first increasing the temperature from 70 190℃ at the rate of 8oC/minute and then isothermal for 10 minute at 190℃. The injector and detector temperature were 250℃ and 300℃; respectively. Carrier gas: Nitrogen at the rate 30 ml/minute, hydrogen and air flow rate were 33 and 330 ml/minute; respectively. The chart speed was 0.4 cm/minute. Peak identifications were established by comparing the retention times obtained with standard methyl esters. The areas under chromatographic peak were measured with electronic integrator as mentioned by[36].
3. Results and Discussion
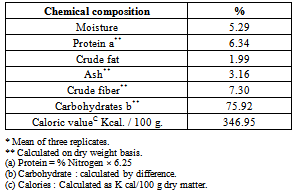
3.1. Gross Chemical Composition and Caloric Value
- Carob powder had been considered as a food supplement in various cultures and it is eaten for their edibility and delicacy. Carob powder fall between the best vegetables and animal protein source[1, 2 and 3].
|
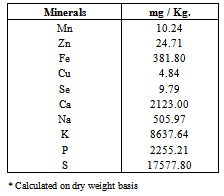
3.2. Minerals Content
- The data of the average values of minerals content in carob powder are outlined in Table (2).
|
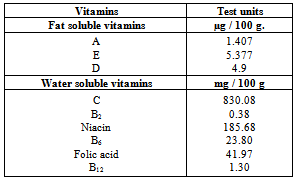
3.3. Vitamins Content
- The data outlined in Table (3) represented the mean values of vitamins content in carob powder. The data revealed that carob powder is a good source of vitamins E, D, C, Niacin, B6 and folic acid. Meanwhile, carob powder contained lower levels of vitamins A, B2 and B12. Such data are in agreement with[38].
|
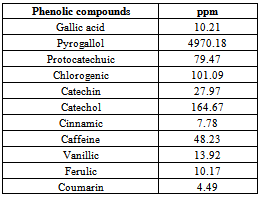
3.4. Phenolic Compounds Content
- The phenolic compounds content of the carob powder is presented in Table (4). The data revealed that the phenolic compounds of the carob powder consisted of 11 compounds. Phrogallol, catechol, chlorogenic, and protocatechuic recorded the highest values, while coumarin, cinnamic, ferulic, gallic acid, and vanillic recorded the least values of the phenolic compounds. The data are in agreement with[40, 41 and 42]. Both chlorogenic acid and caffeic acid are antioxidants and inhibit the formation of mutagenic and carcinogenic N-nitroso compounds in vitro as reported by[42, 43].Moreover, some phenolic acids (Caffeic acid, ferulic acid, gallic acid, and protocatechinic acid contribute against various types of cancer such as breast, lung and gastric cancer as reported by[44, 45, 46, 1, and 7].
|
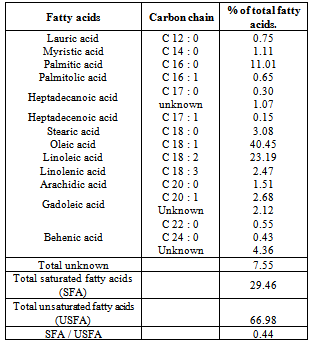
3.5. Fatty Acid Composition
- The fatty acid composition data of carob powder are presented in Table (5). The obtained data revealed that the carob powder oil considered of 17 fatty acids, but consisted mainly of four fatty acids namely: oleic, linoleic, palmitic and stearic recording 40.45%, 23.19%, 11.01% and 3.08%. The data are in good accordance with[26, 3 and 18].The total unsaturated fatty acids recorded 66.98%, while the total saturated fatty acids recorded 29.46% . Besides, Table (5) revealed that the saturated fatty acids / unsaturated fatty acids ratio accounted to 0.44.Generally, there are two most important parameters related to fatty acid composition of any oil, one is the ratio of total saturated fatty acid / total unsaturated fatty acid, which is related to the oxidation stability of the oil, while the second is oleic / linoleic ratio, which has a positive effect on the taste of the oil[47].In conclusion the carob powder is being acclaimed as an ingredient with a marked nutritional value due to its high levels of dietary fibre and phenol compounds. The soluble fibres in particular are thought to exert a preventative role against heart disease, as they appear to have the ability to lower serum cholesterol. Besides the polyphenols have antioxidant activity, which mainly enhances the prevention or delay the oxidative damage. Consequently, polyphenols are involved in protection against several diseases (cardiovascular and neuronal, among others). As a result, carob powder should be increasing interest as an ingredient in the food industry such as functional and healthy foods formulations as Biscuits, bread, and cakes.
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML