-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(6): 289-303
doi:10.5923/j.fph.20130306.05
Recent Applications of Pressurized Fluid Extraction: Curcuminoids Extraction with Pressurized Liquids
J. Felipe Osorio-Tobón, M. Angela A. Meireles
LASEFI/DEA (Department of Food Engineering)/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Rua Monteiro Lobato, 80; Campinas, SP; CEP: 13083-862, Brazil
Correspondence to: M. Angela A. Meireles, LASEFI/DEA (Department of Food Engineering)/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Rua Monteiro Lobato, 80; Campinas, SP; CEP: 13083-862, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Pressurized liquid extraction is a versatile technique that allows the extraction of natural bioactive compounds such as curcuminoids, compounds that have medicinal properties and responsible for the yellow color of turmeric. Generally, used as an analytical tool pressurized liquid extraction uses elevated temperatures (313 – 473 K) and moderate to high (3.5 – 20 MPa) pressures to facilitate and enhance the extraction process. Various features such as the use of smaller amounts of solvents, reduced extraction time and no exposure of the compounds to oxygen and light, give this technique advantages over traditional processes of solvent extraction. This review describes the fundamentals and parameters influencing the process of pressurized liquid extraction, exploring the latest developments and trends in the extraction of bioactive compounds such as curcuminoids. It also discusses the possibility of using near room temperature (313 K) and pressures in the range of 10 – 30 MPa as opposing to the use of high temperatures, in the extraction of pigments from plant material rich in starch.
Keywords: Pressurized Fluid Extraction, Extraction parameters, Bioactive compounds, Curcuminoids
Cite this paper: J. Felipe Osorio-Tobón, M. Angela A. Meireles, Recent Applications of Pressurized Fluid Extraction: Curcuminoids Extraction with Pressurized Liquids, Food and Public Health, Vol. 3 No. 6, 2013, pp. 289-303. doi: 10.5923/j.fph.20130306.05.
Article Outline
1. Introduction
- The increase of scientific knowledge about the impact of foods and synthetic additives on human health and customer awareness that natural compounds can replace those obtained synthetically has generated interest in the development of new processes that allow the extraction of bioactive compounds from natural matrices.Turmeric (Cúrcuma longa L.) is a plant widely cultivated in countries and regions with tropical and subtropical climates, mainly in China, India and Indonesia, as well as in some Latin American countries such as Brazil and Peru, for instance[32]. Turmeric has been used popularly as a preservative, flavoring and coloring agent. The yellow color of the rhizomes is due to the presence of a group of phenolic compounds called curcuminoids. Turmeric has been investigated owing to its benefits on human health and their bioactive properties, such as: anti-inflammatory, antioxidant, chemopreventive and chemotherapeutic activity[27].Nowadays, the curcuminoids extraction for use as potential natural food additive is made using conventional solvent extraction techniques such as Soxhlet and solid- liquid extraction. In conventional methods of extraction, factors such as light, temperature and exposure to oxygen produce curcuminoids degradation[69], limiting and restricting the application processes by which they are obtained. This aspect combined with the low yield and high cost of operation of conventional processes highlights the need to implement new processes to overcome these drawbacks and also protect the integrity of the compounds and generate environmentally friendly process. Pressurized liquid extraction (PLE) is an attractive alternative because it allows fast extraction and reduced solvent consumption[60]. PLE has frequently been used for analytical purposes in the preparation of samples, overcoming disadvantages of the conventional methods of extraction. It is a technique characterized by being easily automated, making it a distinctive technique with low cost and favorable environmental impact because of low solvent usage[81].PLE is performed in a wide range of conditions on the liquid compressed region, located to the left of the saturated liquid curve and below the critical temperature (Tc) line, as shown in the pressure-volume diagram for ethanol (Figure 1). In this region the liquids are highly incompressible and when the solvents are subjected to pressure changes at a constant temperature, their density and solvation power are not affected significantly[73]. However increased temperatures improve the efficiency of extraction because of enhanced rate of mass transfer and diffusion rates[60].The aim of this article is to provide an overview of the fundamentals and parameters governing pressurized liquid extraction. As well as to explore the latest trends and applications that involves the use of PLE, focusing on the extraction of bioactive compounds such as curcuminoids from natural matrices.
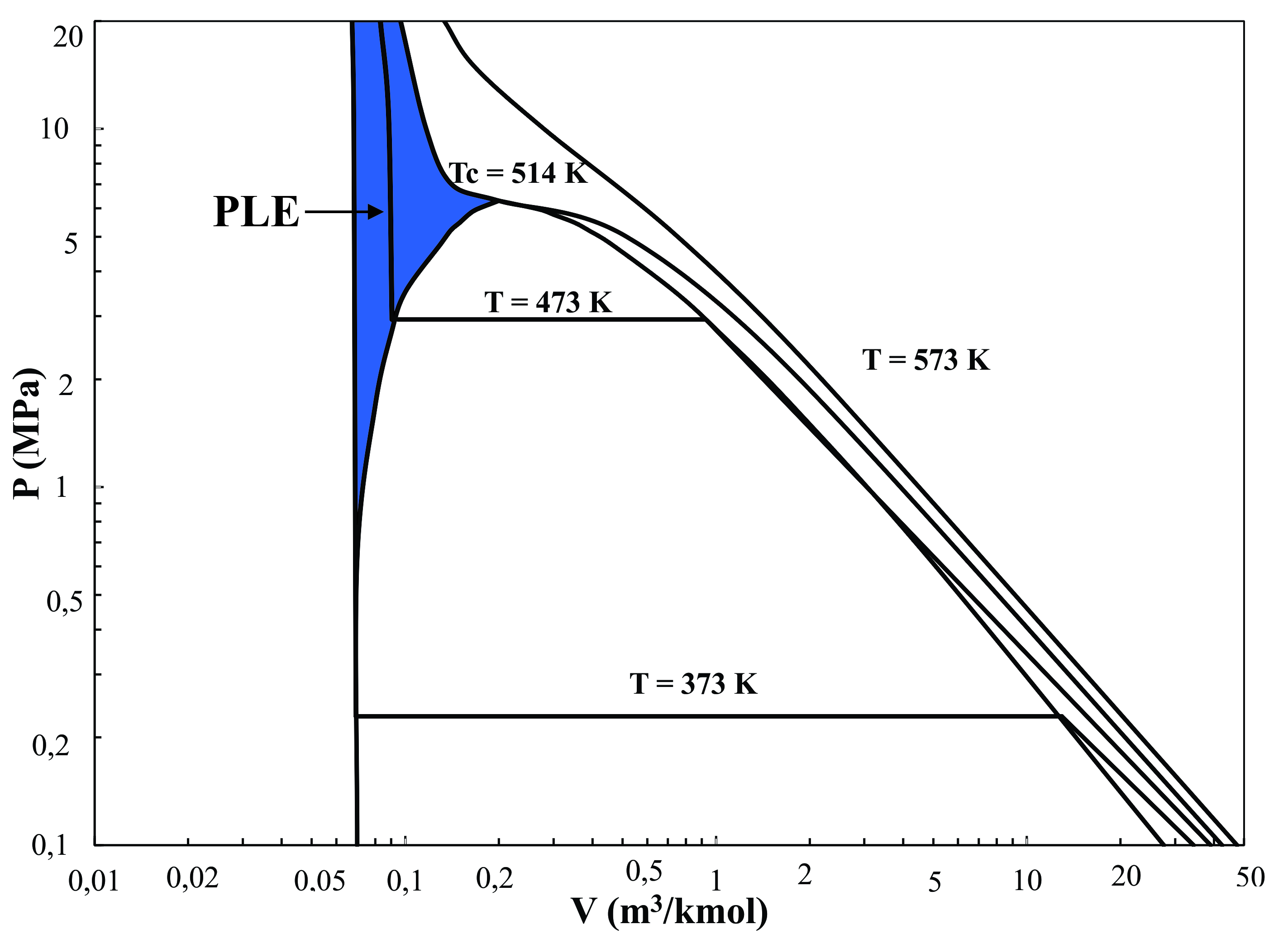 | Figure 1. Pressure-volume diagram for ethanol calculated using the Peng – Robinson equation of state |
2. Curcuminoids
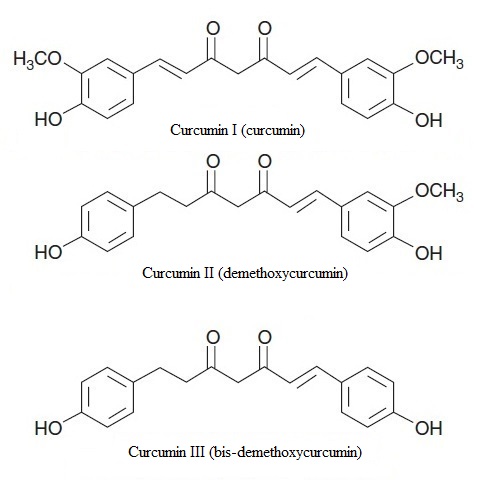 | Figure 2. Chemical structures of curcuminoids |
2.1. Curcuminoids Extraction
- Classical techniques, such as liquid–liquid extraction, sonication, Soxhlet extraction, and related methods have been used traditionally in the curcuminoids extraction[22]. The traditional process involves the preparation of the raw material (milled and dried), extraction with a suitable solvent (acetone or methanol) which selectively extracts the coloring matter, extract purification and finally remove the solvent. This process, after distillation of the solvent, yields an oleoresin with coloring matter content of approximately 25-35 % along with volatile oils (15-20%) and other resinous extractives (20-30%). The oleoresin so obtained is subjected to further washes using selective solvents that can extract the curcumin pigment from the oleoresin. Depending on the size of the batch and of the extraction parameters, each batch takes between 6 to 24 hours. This process yields a powdered, purified food color, known as curcumin powder identified with the food code E100, with over 90 % of coloring matter content and very little volatile oil and other dry matter of natural origin[71].The selection of solvents is done with care to meet solubilization and regulatory criteria. Curcuminoids are poorly soluble in water, but they are soluble in ethanol, alkali, ketone, acetic acid and chloroform[15]. According to the European Commission directive 45/CE[13] the following solvents are considered suitable for curcuminoids extraction: isopropanol, ethyl acetate, acetone, carbon dioxide, methanol, ethanol and hexane.
3. Pressurized Liquid Extraction
- PLE is called pressurized fluid extraction (PFE), accelerated solvent extraction (ASE), pressurized liquid extraction (PLE), pressurized solvent extraction (PSE) or enhanced solvent extraction (ESE)[44; 47]. When the solvent used is water, it is common to use other terms such as subcritical water extraction (SWE), hot water extraction (HWE), pressurized hot water extraction (PHWE), high-temperature water extraction (HTWE) superheated water extraction or hot liquid water extraction[11].PLE uses elevated temperatures (313 – 473 K) without reaching the critical point to increase the kinetics of the extraction process while applying high pressures (3.5 – 20 MPa) to maintain the solvents in their liquid state[79]. Depending on the temperature at which the extraction is performed, PLE is a technique suitable for processes where compounds that may be sensitive to degradation through the action of the heat[5]. Furthermore, because the extractions are carried on a closed extractor, the contact of bioactive compounds with oxygen and light is avoided, thus preventing the degradation of those compounds susceptible to degradation by oxidation.The kinetics of the extraction process with pressurized liquid is characterized by an overall extraction curve (OEC), whose kinetic parameters can be calculated by adjusting a linear spline model as used in supercritical fluid extraction (SFE)[12], which is presented in Figure 3. In the OEC can be distinguished mainly three stages (Figure 3): a first stage called constant extraction rate period (CER), followed by a falling extraction rate period (FER) and finally a diffusion controlled rate period (DC). In the PLE process the solvent is always in excess relative to the compound to be extracted. In the CER stage there is a large amount of compound available for extraction, allowing a rapid removal of compounds dissolved in the bulk solution or adsorbed at the surface of the matrix. Subsequently the extraction speed decreases at the FER period when the compounds dissolved in the solvent and/or adsorbed at the pore surface and those dissolved/adsorbed in the micro/nano pores of the matrix are extracted. Finally the extraction rate decreases almost entirely on the DC period when which begins the extraction of compounds that are chemically bonded to the matrix, the matrix-compound chemical interactions must be broken and the compound must be solvated by the solvent and subsequently released to outside the matrix in to the bulk solvent[54].
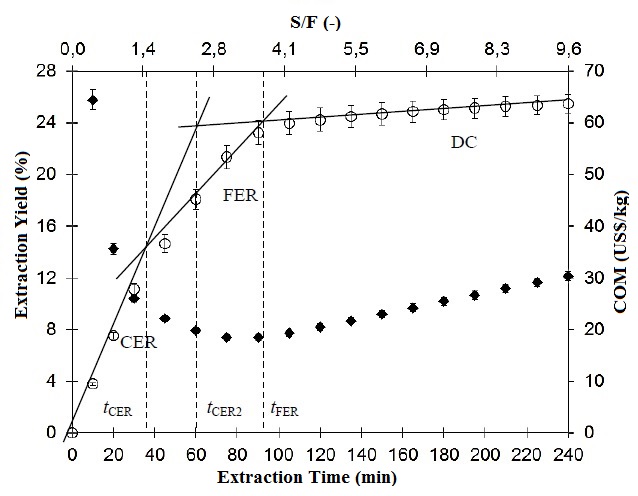 | Figure 3. Overall extraction curves of Jabuticaba obtained by pressurized liquid extraction using ethanol as solvent[12] |
3.1. Description of the Extraction Process
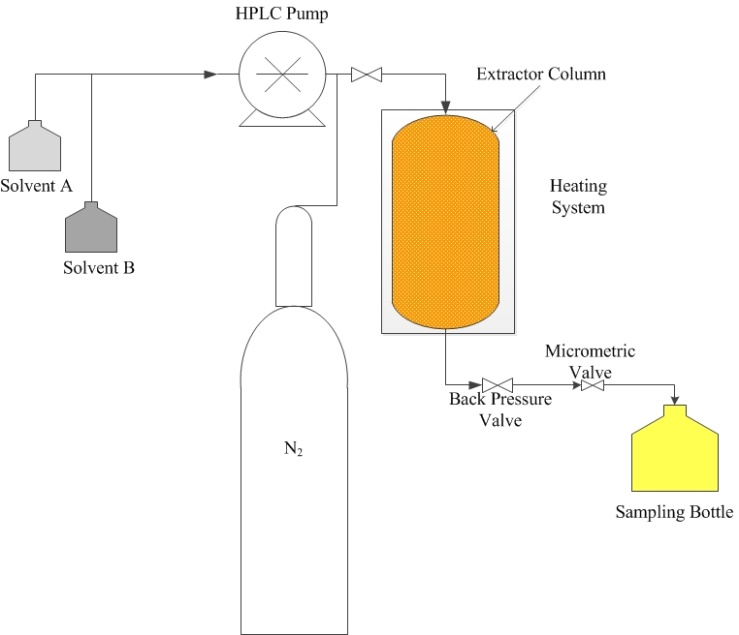
- PLE can be performed in either static or dynamic mode. Static extraction mode is a batch process consisting of one or more extraction cycles with addition of fresh solvent between each cycle. The extractor is pressurized through the solvent inlet while the outlet valve is kept closed. After the extraction, the valve is opened, releasing a mixture of solvent and extract to the collection collecting.In the dynamic mode, the solvent is continuously pumped through the extractor containing the matrix, whereas the outlet valve is kept open during the extraction[11; 54]. Although one would think that the dynamic extraction may produce better results than the static extraction, avoiding the solvent saturation, by injecting continuously fresh solvent into the extractor, the static extraction is more efficient because it allows greater penetration of the solvent into the nano and micro pores of the matrix according to Nieto et al.[48].A basic static pressurized liquid extraction setup is shown in Figure 4. The extraction process involves packing the sample into the extractor and then the solvent is pumped by a HPLC-type pump into the extraction cell, which was placed in a heating system at a desired temperature, until the required pressure was obtained. Once the temperature and pressure of the process have been reached, the extraction is performed over a preset time. If the process has more than one extraction cycle, the solvent is replaced with fresh solvent, in each extraction cycle. After the extraction cycles have finished, the back pressure valve and micrometric valves are carefully opened, keeping the pressure at an appropriate level for the desired flow. Finished the preset extraction time, the HPLC pump and the heating system are turned off. The extractor may or may not be purged using an inert gas such as nitrogen to remove the solvent remaining within the extractor[54; 10].
4. Extraction Parameters
- The PLE process performance is governed mainly by the choice of solvent, temperature, extraction time and to lesser extent by the pressure. However the performance of the process depends on the matrix nature, the specific features of the target compounds and their localization inside the matrix[47]. Therefore it is necessary to know and establish the influence of these factors on the extraction process in order to obtain high yields and high purity extracts. Next, an overall review of the parameters that influence the PLE process is presented, the differences between analytical and process applications are also considered.
4.1. Analytical Applications
4.1.1. Solvent
- The extraction solvent must be highly selective, with high solvation capacity of the target compound and minimize the co-extraction of other matrix components. The polarity of the solvent should be close to that of the target compound. Non-polar solvents such as n-hexane and pentane or a non-polar with medium-polarity solvents, such as pentane/dichloromethane or cyclohexane/ethyl acetate, have frequently been used in the extraction of apolar and lipophilic compounds. On the other hand, more polar solvents, such as acetonitrile, methanol, ethyl acetate or water, have been employed in the case of polar and hydrophilic compounds [11]. Other important solvent characteristic is its ability to aid in the release of compounds from matrix and helping with the breaking of the interactions matrix-compounds[57].Among the most commonly used solvents in the extraction of bioactive compounds from natural sources are: ethanol[41; 46; 50; 60; 82], methanol[30; 35; 40; 68; 65] and n-hexane [16; 81; 31; 36; 55]. When water is used as solvent[9; 22; 55; 62], the combination of high temperatures, pressure and pH changes, contribute to the change of the water polarity, modifying its dielectric constant (ε). At ambient pressure and temperature, water is a polar solvent with a high dielectric constant (ε = 78) but at 573 K and 23 MPa this value decreases to 21, which is similar to the value for ethanol (ε = 24 at 298 K) or acetone (ε = 20.7 at 298 K). This means that at elevated temperatures and moderate pressures the polarity of water can be reduced considerably; thus, water can act as if ethanol or acetone were being used[11].
4.1.2. Temperature
- Temperature is the main parameter responsible of the extraction process acceleration. High temperatures decrease the solvent viscosity, helping with its penetration inside the matrix and consequently, improve the extraction process. Furthermore, elevated temperature decreases the surface tension of the solvent, compounds and matrix and therefore enhances the solvent wetting of the matrix; therefore, to a higher contact between the solvent and those compounds inside the matrix[47]. The use of high temperatures increases the diffusion coefficients, increasing the mass transference rates, furthermore helps to disrupt the compounds–matrix interactions[10; 48]. Nonetheless, for some applications moderate to low temperatures maybe preferred instead in order to avoid some modifications in the solid matrix such as, for instance, the gelatinization of starch in starch-rich solid substratum.
4.1.3. Extraction Time
- The duration of the static extraction time is important in the extraction efficiency since prolonged contact periods between the matrix and the solvent permits increased swelling with enhanced matrix wetting and increased penetration of solvent into the nano and micro pores with a greater solvation of compounds. Therefore, an enhanced possibility of the solvent breaking specificcompounds–matrix interactions is ensured[57]. Generally, for analytical applications, extraction times between 5 and 30 minutes are enough to guarantee the extraction of the most compounds with a high yield. However, the combination of high temperatures and long extraction times could induce the degradation of the compounds and the matrix[54].
4.1.4. Pressure
- Pressure is a parameter that does not present big influence on the yield of extraction process, because liquids are not compressible fluids; therefore, even under large pressure changes the solvation power of the solvent is not significantly affected. This effect was observed by Rizvi[54] for pressures between 3 and 20 MPa. Nonetheless, when the vapor pressure of the target components is important for its solubilization in the solvent, pressure may have an important role in the PLE process. Otherwise, the use of high pressures facilitate the extraction of compounds located inside the matrix pores, due to a pressure increase which forces the solvent to penetrate into places which are normally not reached by the solvent at atmospheric pressure[33]. Depending of the structure of the matrix and the particularities of each process, the use of high pressures could be a positive or negative influence on the extraction process, for instance, at higher pressures; the matrix may be compacted, affecting the flow of the solvent[37].
4.2. Process Applications
4.2.1. Particle Size
- It is important to determine the influence of particle size on the extraction process due to the fact that different size fractions are obtained by passing the milled material through a nest of sieves and extracting each fraction. According to Cheah et al.[16], the particle size exert a significant influence on the performance of PLE process, owing to the smallest particle size generate a greatest percentage of extractable solids allowing a greater recovery of bioactive compounds. Particle size reduction by milling not only increases the specific area of raw materials but also ruptures cell walls, releasing a greater amount of bioactive compounds[75].
4.2.2. Solvent
- Aspects such as economy, safety and sustainability must be considered to choose the solvent. Less toxic and non-harmful solvents that are easy to remove or recover should be preferred[47]. Larger volumes of expensive and toxic organic solvents have to be used in many cases, which is not applicable for food industry; additionally, toxic solvent disposal is expensive. In recent years, continuous efforts have been made in order to reduce the amount of organic solvents required in extraction process. With continuous interests and investment in biofuel industry, ethanol has become the cheapest solvent after water. Different from industrial ethanol synthesized from petroleum, ethanol produced by fermentation is listed as safe, clean, green and sustainable[29].
4.2.3. Temperature
- Temperature is an important parameter and usually the higher yields obtained with PLE in comparison with other extraction techniques are attributed to this parameter.An increase in extraction temperature is reported to improve the efficiency of extraction because of enhanced diffusion rate; nevertheless, a maximum temperature limit should be fixed and should depend on other factors, particularly extraction time[60]. The stability of the compounds of interest and the matrix can be affected by the combination of high temperatures and pressures, therefore, the process temperature must be carefully selected so as to not cause degradation of bioactive compounds or the matrix, or the increased extraction of other undesirable compounds [29; 18].
4.2.4. Cost of Manufacturing (COM)
- Although when PLE is compared with traditional extraction methods, the PLE process appears as a valuable alternative in terms of extraction, the economic viability of the process is very important and even more when considering that there are other technologies that have a lower investment cost[51]. The PLE process appears to be a technically promising and economically viable technique. Santos et al.[59] determined the cost of manufacturing (COM) for the extraction of phenolic compounds from Jabuticaba skins obtained under the optimum PLE conditions were 40-fold lower in comparison with a conventional low-pressure solvent extraction (LPSE) for a process time of 2 h.
5. PLE Applications
- The increasing appearance of degenerative diseases has increased consumer concern regarding the use and ingestion of foods. In this sense, the use of vegetable extracts with functional characteristics (specific physiologic benefits due to the presence of bioactive substances), with high purity (synthetic substances, often, are suspected to cause secondary effects to health) has attracted the interest from institutions and companies involved in the formulation of food and/or products with beneficial health effects.A literature survey was conducted regarding the use of PLE in the area of food science and technology considering analytical and process applications. A survey was conducted in Web of Science and Scopus databases considering the period between 2006 and 2013, several articles were found about the utilization of PLE in the area of food science and technology.
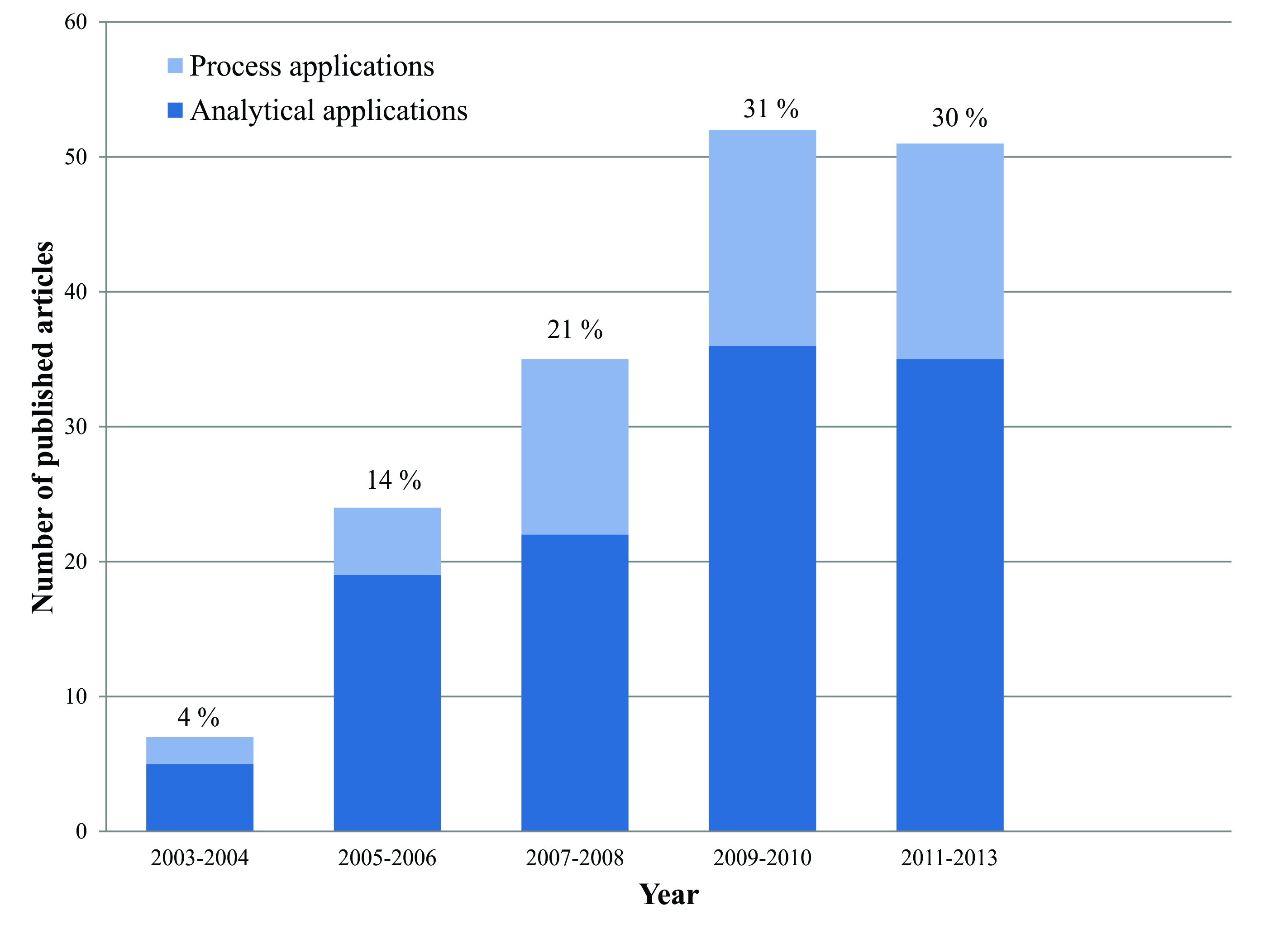 | Figure 5. Distribution of the published articles using PLE in the area of food science and technology |
5.1. Extraction of Bioactive Compounds from Natural Matrices
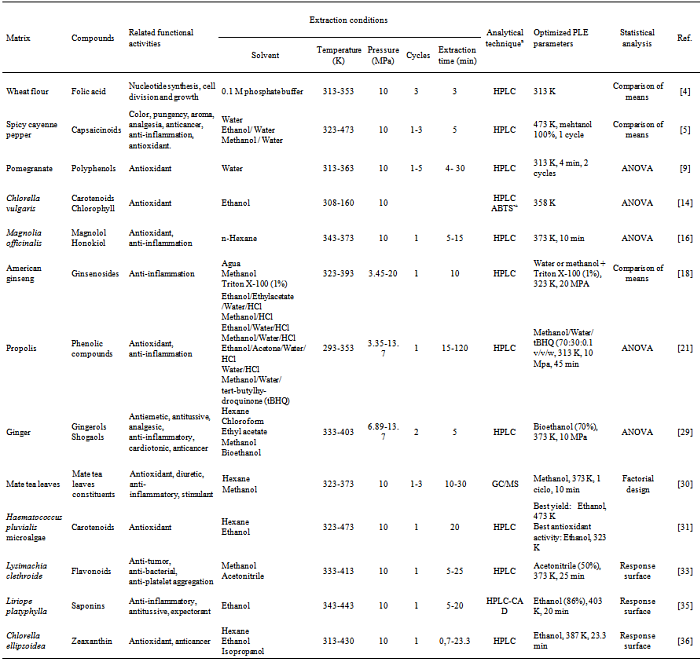
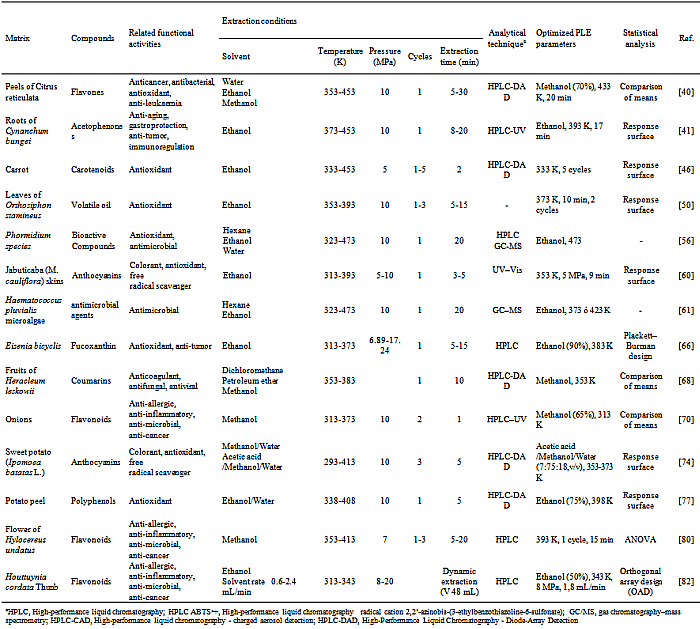
- PLE has been utilized in extraction of several bioactive compounds such as flavonoids[70; 82], polyphenols[9] and several antioxidants compounds[77; 74] from natural matrices such as plants, fruits, vegetables and microorganisms as showed in Table 1.Exhaustive extraction of honokiol and magnolol from Magnolia officinalis were studied by Cheah et al.[16]. PLE is a more economical alternative to Soxhlet in exhaustive extraction as it requires less solvent and time. The proportions of active principles in the extracts obtained by PLE were higher compared to Soxhlet (honokiol 5% vs 3%; magnolol 46% vs 30%). Only 20 minutes of PLE with 5 minutes of static time were enough to extract almost the totality of compounds extracted with Soxhlet after 8 hours of extraction[29].PLE extraction of flavones was investigated and compared with other conventional extraction techniques such as ultrasonic-assisted extraction (UAE), heat-reflux extraction (HRE) and Soxhlet extraction. PLE and HRE. Although the efficiencies of HRE and PLE for flavones extraction were similar, PLE method was less time-consuming. The PLE method required only 20 min under 433 K to complete the extraction while Soxhlet, UAE and HRE required extraction times of 4 hours, 1 hour and 20 minutes, respectively[40].Additionally, PLE was utilized for acetophenones extraction, 17 minutes static extraction time were necessary to overcome the extraction yield of HRE and Soxhlet, which took between 6 and 9 hours, respectively, to be completed[41]. PLE extraction of phenolics compounds from Jabuticaba (M. cauliflora) was compared to LPSE by Santos et al.[60], under optimized conditions both extraction techniques presented similar yield, however, PLE extracts showed in their composition higher content of anthocyanins and phenolics compounds. Likewise, the cost of manufacturing (COM) obtained for the PLE extract was 40-fold lower than conventional low-pressure solvent extraction (LPSE).Flavonoid extraction from onions were studied by Søltoft et al.[70] comparing PLE with Microwave-assisted extraction (MAE) and using a ultrasonic liquid processor (ULP). Although the efficiencies of MAE and ULP were comparable and at the same level as PLE, MAE and ULP needed an additional sample preparation step (e.g. centrifugation). Likewise, difficulties were observed during the filtration of extracts MAE and ULP. In contrast, PLE yielded clean extracts, which could be filtered directly after extraction.Due to growing interesting in nutraceutical and bioactive compounds extraction from natural fonts and the parallel concerns about use of technologies that are more "green", PLE is becoming a promising extraction technology to satisfy these requirements[10].
5.2. Detection of Contaminants and Toxic Substances in Foods
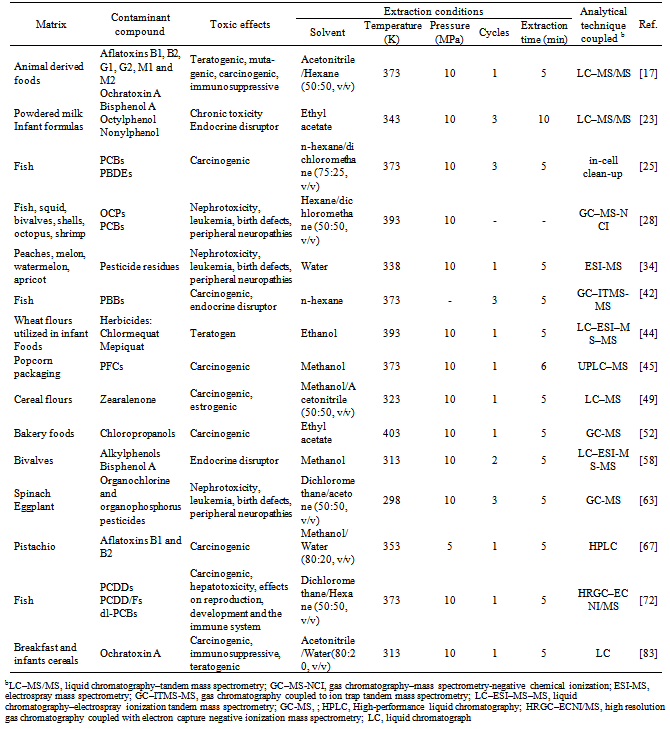
- PLE coupled with other analytical techniques that have been used in extraction and quantification of contaminants compounds presents in several food stuffs such as fruits, vegetables, meats and cereals (Table 2).In articles published in recent years, it is possible to observe that PLE application in the analysis of contaminants in foods has been mainly focused on detection of organic compounds used in plastic manufacturing[23], pesticides[28] and mycotoxins[17].Since the food matrices are complex and contaminants are at a trace level in the matrices, it requires investing a large amount of time in the conditioning and sample preparation. However, in analytical applications, PLE is a robust and time-saving technique that would enable automated sample-handling and avoid health risks caused by both the contaminants compounds and the solvents[17].In the food analysis by PLE a dispersing agent is frequently used for improving the interactions between sample and extraction solvent, as well as ensuring the reproducibility of the extraction. In sample preparation of matrices with high lipid content, lipophilic sorbents are widely employed, for instance. To avoid this, neutral alumina, florisil, graphitized carbon (120/400 mesh) and amino propyl silica were tested[58].Bisphenol A (BPA), is a endocrine disrupting chemical. This product is used in the manufacture of epoxy resins and polycarbonate plastics, which are, in turn, used in a wide variety of domestic products such as in dental fillings, plastic food and water containers, baby bottles, food wrap, as well as in the lining of beverage and food can[20]. Several authors have used PLE as a method for detection and quantification of these compounds[20; 23; 58]. Temperature is an important factor in bisphenols extraction, an increase in temperature has a negative effect on the recoveries of analytes, because higher amounts of matrix components that are extracted at higher temperatures affect the quantification of the analytes. For instance, Ferrer et al.[23] developed a method for quantifying the bisphenol contents in powdered milk and infant formulas. The recoveries of BPA at temperatures above 343 K were lower. A temperature of 393 K gave the worst results, because an increase of the temperature resulted in a cloudy extract with an increasing amount of material co-extracted from the matrix that hampered the quantification of the compounds. On the other hand to avoid the amount of unwanted compounds Salgueiro-González et al.[58] used an extraction temperature of 313 K for the analysis of BPA in bivalve molluscs. Besides the influence of temperature on the extraction and quantitation of BPAs, the choice of solvent is an important factor, since due to the BAPs low solubility in water is recommended to use organic solvents such as ethyl acetate and methanol, because the presence of water is responsible for low yields in the extraction[20].Due to excessive use of agrochemical in agriculture, have been detected appreciable levels of these chemicals in soil, water and food. Those contaminant substances have been entered in food chain of customers, accumulate in the body with the consequent generation of negative effects.Several PLE methods have been validated to pesticide detection, for example: organochlorine and organophosphorus pesticides[28; 63; 24], polychlorinated biphenyls (PCBs)[25; 39] and polybrominated biphenyls (PBBs)[42]. Temperature has a fundamental role in the extraction process and quantification of contaminant compounds, and as in the detection of other contaminants, an increase in temperature, produces a large amount of additional peaks in chromatography techniques coupled to extraction, preventing the identification of the contaminant compounds[24].PLE detection of mycotoxins has had an important development, with successful application in detection of contaminants in different food, making it a potentially useful tool to measure with accuracy the levels of mycotoxins. The mycotoxin Zearalenone (ZEN) is produced by several fungi, including Fusarium graminearum and Fusarium culmorum in cereal flours and is associated with development of breast cancer, was analyzed by Pérez-Torrado et al.[49] using PLE coupled with liquid chromatography–mass spectrometry (LC-MS), achieving European Commission level requirements. Aflatoxins produced by Aspergillus flavus and Aspergillus parasiticus, highly toxic and carcinogenic, can be extracted by PLE, presenting higher extraction yield or recovery in comparison with the AOAC method using similar extraction solvent[67]. On the other hand, Zinedine et al.[83] and Chen et al.[17] used PLE to extract ochratoxin A in cereals. Ochratoxin A is known to cause a wide range of toxic effects due their nephrotoxic and carcinogenic. The PLE methods coupled with LC-MS demonstrated their ability to extract quickly and automatically residues of mycotoxins, allowing great accuracy over a wide range of concentrations meeting the criteria established by the European Commission.
|
|
|
6. Curcuminoids Extraction by PLE
- The need to develop more efficient processes, avoiding the massive use of organic solvents for the extraction of curcuminoids, has allowed the development of more rapid and environmentally friendly techniques.After a literature survey in Web of Science and Scopus databases it was found several articles about curcuminoids extraction using various techniques. The extraction of curcuminoids by MAE[43; 76] and supercritical fluid extraction (SFE)[6; 7; 26; 76] was studied.But, more specifically about curcuminoids extraction using pressurized fluids only 3 articles were found. Braga and Meireles[8] studied SFE using a high percentage of cosolvent into the extraction system, the process was denominated accelerated solvent extraction (ASE). The cosolvent (EtOH/IsoC3, 1:1 v/v) percentages used were 10, 50 and 90% (v/v); however, the higher yield of curcuminoids was obtained when a concentration of 50% cosolvent was used at 313 K and 30 MPa for 30 minutes of static extraction.Schieffer[64] compared PLE with ultrasonically assisted extractions. Methanol was used as solvent at 373 K, 10 MPa and 5 minutes of static extraction time. PLE demonstrated higher performance, due to the fact that ultrasonically assisted extraction left a small amount, but significant, of curcuminoids in the sample. Euterpio et al.[22] used water as solvent and due to the low solubility of the curcuminoids in water, was necessary to increase the extraction temperature to improve the extraction. However, at temperatures above 473 K, both curcumin and turmeric matrix were rapidly degraded and the extracted rhizome particles took on a deep dark brown color resulting in poor extraction yields. This situation was improved by acidifying the water (pH 1.6) and using phosphate buffer (62 g/L), which increased the solubility of curcuminoids in a dynamic extraction process with solvent rate of 0.5 mL/min at 370 K and 5 MPa.Although curcuminoids have many potential uses in industry, until now the study by Braga and Meireles[8] has been the only study to use pressurized liquids in order to study the effect of the extraction parameters of the process of separation of the volatile oil and oleoresin using fractionated SFE with different solvents, in order to maximize the extract yield and curcuminoid content.
7. Conclusions
- This review has shown that PLE is a highly selective process that uses short periods of extraction and small amounts of organic solvents. PLE is presented as an excellent alternative to replace the conventional techniques of extraction, to have a potential use in the extraction of bioactive compounds and the assessment of food contaminants when it is coupled to an analytical technique.Likewise, PLE represents a good alternative for extraction of curcuminoids, ensuring high extractability, producing high purity extracts in conditions that avoid the degradation of curcuminoids.However, there is still a lack of studies (only 3 articles were found in the journals indexed in the Web of Science and Scopus databases) that allow establish the influence of different parameters on the extraction process of curcuminoids using PLE and determine the best conditions for the extraction process, which in turn allows to study the viability and scale up process to implementing a production line of curcuminoids extracts using PLE.
ACKNOWLEDGEMENTS
- Authors are grateful to CNPq (470916/2012-5) for the financial support; partial support from FAPESP (2012/10685-8) is also acknowledged. J. Felipe Osorio-Tobón thanks CAPES for the PhD assistantship. M. A. A. Meireles thanks CNPq for the productivity grant (302778/2007-1).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML