-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(6): 277-283
doi:10.5923/j.fph.20130306.03
Microbiological and Physico-Chemical Quality of Smoked Shrimp, An Expanding Food Condiment in Beninese Local Markets
Euloge Y. Kpoclou1, Victor B. Anihouvi1, Paulin Azokpota1, Mohamed M. Soumanou2, Georges Daube3, Caroline Douny4, François Brose4, Marie-Louise Scippo4, D. Joseph Hounhouigan1
1Department of Nutrition and Food Science, Laboratory of Microbial Biochemistry and Food Biotechnology, Faculty of Agronomic Sciences, University of Abomey-Calavi, 01 BP 526, Cotonou, Benin
2Research Group on Enzyme and Food Engineering, Laboratory of Study and Research in Applied Chemistry, Polytechnic School of Abomey-Calavi, University of Abomey-Calavi , 01 BP 2009, Cotonou, Benin
3Departement of Food Science, Laboratory of Food Microbiology, Faculty of Veterinary Medicine, University of Liège, Boulevard de Colonster 20, B-4000 Liège, Belgium
4Departement of Food Science, Laboratory of Food Analysis, Faculty of Veterinary Medicine, Centre of Analytical Research and Technology (CART), University of Liège, Boulevard de Colonster 20, B-4000 Liège, Belgium
Correspondence to: Euloge Y. Kpoclou, Department of Nutrition and Food Science, Laboratory of Microbial Biochemistry and Food Biotechnology, Faculty of Agronomic Sciences, University of Abomey-Calavi, 01 BP 526, Cotonou, Benin.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
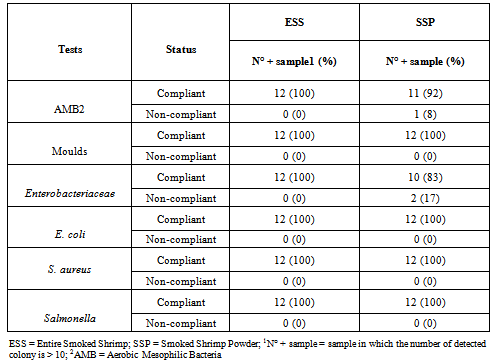
Entire Smoked Shrimp (ESS) and Smoked Shrimp Powder (SSP) are two food condiments widely used in Beninese local cooking practices. Twelve samples of each product collected from local markets were evaluated for safety assessment using standard methods. Regarding the microbiological status of the samples, the Enterobacteriaceae were detected in 83% and 75% of ESS and SSP respectively, whereas 25% of samples of each product were found to contain E. coli. Pathogenic bacteria such as S. aureus and Salmonella were absent. Except 8% and 17% of SSP sample exceeding the maximal limit of 106 UFC/g for Aerobic Mesophilic Bacteria and 104 UFC/g Enterobacteriaceae respectively, all the other samples were within the acceptable limits. Water activity values were low, ranging between 0.54±0.01 for SSP and 0.61±0.01 for ESS, showing a potential microbial stability. Considering the chemical hazards, 15 EU priority polycyclic aromatic hydrocarbon (PAHs) were detected in the samples examined with median Benzo(a) pyrene and PAH4 contents (91 μg kg-1 and 490 μg kg-1respectively) exceeding the European maximal limit (5.0 μg kg-1 and 30 μg kg-1). This study showed that smoked shrimps may be generally safe from a microbiological point of view, but they constitute a large source of exposure to possible carcinogenic PAHs.
Keywords: Shrimp, Smoking, Polycyclic Aromatic Hydrocarbon (PAH), Microbiological Quality, Market
Cite this paper: Euloge Y. Kpoclou, Victor B. Anihouvi, Paulin Azokpota, Mohamed M. Soumanou, Georges Daube, Caroline Douny, François Brose, Marie-Louise Scippo, D. Joseph Hounhouigan, Microbiological and Physico-Chemical Quality of Smoked Shrimp, An Expanding Food Condiment in Beninese Local Markets, Food and Public Health, Vol. 3 No. 6, 2013, pp. 277-283. doi: 10.5923/j.fph.20130306.03.
Article Outline
1. Introduction
- In many tropical countries, the fishing surpluses are processed to be used as food condiments[1-4]. In Benin, smoked shrimp is a food condiment widely used in local cooking practices[5, 6]. Post-harvest processing of shrimp is essentially assumed by women of fishing communities. Shrimps are processed by artisanal hot smoking method to obtain a dry product. Then, they are stored in a basket at ambient temperature (30-33°C). Furthermore, smoked shrimps after solar drying (facultative) are ground and packaged in bottles or plastic bags. In Beninese traditional hot smoking practice, shrimps are in direct contact with wood smoke[7]. During smoking process, polycyclic aromatic hydrocarbons (PAHs) can be formed from the organic matter such as firewood[8]. More than 300 congeners constitute the PAHs family, among which 15 have been recognized as genotoxic by the European Union (EU) [9,10]. The benzo(a)pyrene has been recognized as carcinogenic for humans[11] and 6 other PAHs (benzo [a] anthracene, chrysene, benzo[b]fluoranthene, benzo [k] fluoranthene, dibenzo[a,h]anthracene, and indeno[1,2,3-cd] pyrene) have been classified as probable human carcinogens [12]. Furthermore, the microbiological quality of foods often reflects the hygienic status of the region where they are produced and manufactured. It is evident that as many condiments such as fermented fish[13, 14] and spices[15-17], smoked shrimps are exposed to a wide range of microbiological and chemical contaminations during the catch, the processing, the storage and in the retail markets. Previous studies have reported the presence of toxigenic moulds and mycotoxins such as aflatoxins and ochratoxins in smoked dry fish collected in retail markets in tropical conditions[18, 19]. Smoking treatment could not destroy all kind of micro-organisms and wood smoke is a potential source of many toxic contaminants. Thus, smoked shrimps may be considered as a potential vehicle for transmission of food borne diseases. The present work aims to assess the safety of smoked shrimps as sold in Beninese retail markets.
2. Materials and Methods
2.1. Samples Collection
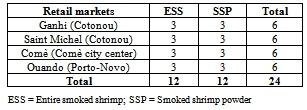
- A total of 24 samples comprising 12 samples of ESS and 12 other samples of SSP were randomly purchased from retail markets of Ganhi and Saint Michel (Cotonou), Comè (Comè city center) and Ouando (Porto-Novo) (Table 1). The entire smoked shrimps usually sold in bulk were collected in sterile stomacher bags while samples of smoked shrimp powder were collected with their glass package. Samples were transported to the laboratory within 2 h for immediate microbiological, pH and water activity analysis, or stored à -20°C until other chemical analysis.
|
2.2. Microbiological Analysis
- Twenty-five gram (25 g) of each sample was suspended in 225 ml of buffer peptone water (Oxoid CM0509B, Basingstoke, Hampshire, England), and homogenized for 2 min using a laboratory blender (Stomacher Lab-Blender 400, model N° BA 6021, Seward, London, UK). Serial decimal dilutions were prepared in buffer peptone water as described by ISO 6887-3[20], and inoculated in different media: (i) plate count agar (PCA, Oxoid CM0463B, Basingstoke, Hampshire, England) for total viable counts; PCA plates were incubated at 30°C for 3 days[21]; (ii) Baird-Parker Agar Base (BP, Oxoid CM0275B, Basingstoke, UK) for Staphylococcus aureus; BP plates were incubated at 37 °C for 1-2 days, followed by coagulase test[22]; (iii) Violet Red Bile Glucose Agar (VRBG, Oxoid CM0485B, Hampshire, UK) for Enterobacteriaceae; the plates were incubated at 37°C for 1 day, followed by the confirmation of characteristic colonies using oxidase and fermentation tests[23]; (iv) TBX medium (Oxoid CM0945B, Basingstoke, Hampshire, England) for Escherichia coli; TBX plates were incubated at 44 °C for 1 day[24]; (v) Chloramphenicol glucose agar (Biokar diagnostics-zac de ther-allone-F60000 Beauvais) for moulds; the plates were incubated at 25°C for 3-5 days[25]. Results were expressed as colony forming units per gram of sample (detection limit = 10 CFU/g). Qualitative detection of Salmonella was performed by pre - enrichment in Buffered peptone water (37°C; 1 day), and selective enrichment (37°C; 1 day) in Rappaport-Vassiliadis broth (Oxoid CM0669B., Basing-stoke, Hampshire, England) and Muller Koffman broth (Oxoid CM0343B, Basingstoke, Hampshire, England). Cultures were plating-out (37°C; 1 day) on X.L.D medium (Oxoid CM0469B, Basingstoke, UK) and Salmonella, Shigella Agar (Oxoid CM0099B, Basingstoke, Hampshire, England) followed by confirmation of characteristics colo-nies using appropriate biochemical and serological tests for Salmonella[26].
2.3. Physico-Chemical Analysis
2.3.1. Moisture Content, pH and Water Activity (Aw) Determination
- The pH of the samples was determined as described by Goulas and Kontomina[27] using a digital pH-meter (Inolab pH 730 WTW 82362 Wellheim, germany). The dry matter content was determined by oven drying of 5 g of grinded shrimp at 105°C until a constant weight was reached[28]. Water activity (Aw) was measured according to the method described by Anihouvi et al.[13], using a thermo-hygrometer recorder (Rotronic HygroLab 2, 8303 Bassersdorf).
2.3.2. Polycyclic Aromatic Hydrocarbons Determination
- Individual polycyclic aromatic hydrocarbon (PAHs) standard solutions in acetonitrile (ACN) (purity: 98.5–99.9%) were purchased from Cluzeau Info Labo (Putteaux la Défense, France). The deuterated DiP-D14 (in toluene, purity: 99.7%), used as internal standard, was purchased from LGC Promochem (France). Working standard solutions were prepared by dissolving the commercial solutions in acetonitrile and stored at 4°C in dark vials sealed with PTFE/silicone caps.High performance liquid chromatography coupled to fluorescence detector (HPLC/ FLD) analysis was carried out using a Model 600 E solvent delivery system, equipped with a Model 717 automatic injector, a MistralTM oven and both 996 PDA and 2475 Fluorescence detectors (all from WATERS). A C18 Pursuit 3 PAH (100 x 4.6mm, 3µm) equipped with a ChromGuard (10 x 3mm) precolumn, both from VARIAN, were used to separate the PAHs. The PAHs were extracted as described by Veyrand et al.[29]. Briefly, one gram of freeze-dried shrimp sample was extracted with Hexane/acetone (50:50, v/v) using the Accelerated Solvent Extraction (ASE) technique. The solvent was evaporated until 1 ml and reconstituted with 5 ml of cyclohexane. The reconstituted extract was purified by column chromatography using Envi Chrom P column (Supelco) conditioned successively with 15 ml ethyl acetate and 10 ml cyclohexane. After loading the sample extract, the column was washed with 6 ml cyclohexane/ethanol (70:30, v/v) and PAHs were eluted using 12 ml of cyclohexane/ethyl acetate (40:60, v/v). The solvent was evaporated until dryness to change solvent to 90 µl acetonitrile. The final extract was then spiked with 10 µl of deutered DiP (internal standard). Five µl of this final extract was injected on HPLC column as described by Brasseur et al., and Danyi et al.[30, 31]. The limit of quantification of the method was 0.85 µg/kg fresh weight for Benzo(j)fluoranthene and Indeno[1.2.3-cd]pyrene and was 0.21µg/kg fresh weight for all the over PAHs.
2.4. Statistical Analysis
- For data of microbiological analysis, Geometric mean, standard deviation and median was calculated replacing value lower than the detection limit (< 10 CFU/g), by 5 CFU/g. Analysis of data was performed by the test T of student using Minitab 14.1. Statistical significance was set at p < 0.05 and means were separated using SNK (Student, Newman and Keuls) range test.
3. Results and Discussion
3.1. Microbiological Characteristics of Investigated Samples
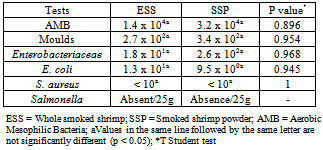
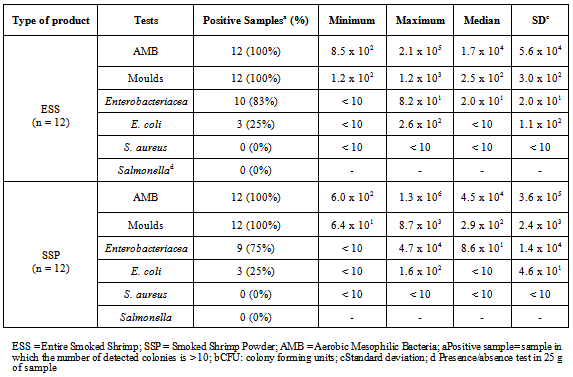
- The geometric means of microbial loads and the description of the contamination level in each kind of product are shown in Tables 2 and 3. No statistical significant difference was noticed through the different evaluated criteria (Table 2). Aerobic Mesophilic Bacteria (AMB) count was up to 1.4 x 104 CFU/g and 3.2 x 104CFU/g in ESS and SSP respectively. Moulds were detected in all the samples examined with a mean value of 2.7 x 102 CFU/g and 3.4 x 102 CFU/g in ESS and SSP respectively. Enterobacteriaceae count was 1.8 x 101 and 2.6 x 102 CFU/g in ESS and SSP respectively. E. coli was detected in 3 samples (25%) of each kind of product up to 1.3x101 CFU/g in ESS and 9.5x100 CFU/g in SSP respectively (Tables 2 and 3). Neither S. aureus nor Salmonella were detected in evaluated samples of the two kinds of product.
|
3.2. Physico-Chemical Characteristics of the Investigated Samples
3.2.1. Moisture Content, pH and Water Activity (aw)
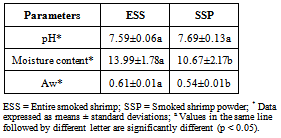
- The pH, moisture content and water activity of the two kinds of smoked shrimp are given in Table 5. The pH values of 7.59 and 7.69 were recorded for ESS and SSP respectively without significant difference (p > 0.05) between the two kinds of product. The moisture content is significantly lower in the SSP (10.67±2.17) than in the ESS (13.99±1.78). The water activity value was also significantly lower (p < 0.05) in the SSP (0.54±0.01) than in the ESS (0.61±0.01). SSP is obtained from the ESS after a complementary solar drying and grounding. The lower moisture content in SSP may be due to this additional drying step of the product. Indeed, Kumolu-Johnson and Ndimele[35] have shown a decrease in moisture content in fish through sun drying. Water activity influences the stability of foods during storage, as some deteriorative processes in foods are mediated by water. According to Prescott et al.[36], bacterial growth would be impossible in food products with a water activity value lower than 0.7. Thus, the low water activity values recorded in the investigated samples during this study are sufficiently low to inhibit the growth of pathogenic bacteria in both smoked shrimps.
3.2.2. PAHs Contents of Smoked Shrimps Investigated
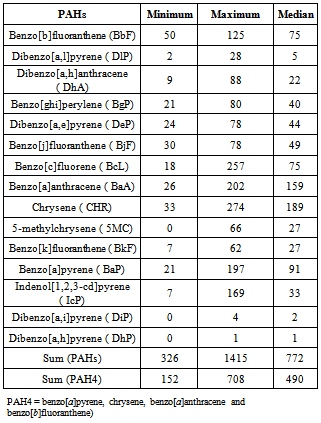
- The results obtained from the PAHs analysis in entire smoked shrimp (ESS) are summarized in Table 6. All the 15 EU PAHs investigated have been detected with a median total PAH concentration of 772 μg kg-1.
|
|
|
|
4. Conclusions
- In the present study, smoked shrimp in its different forms sold in local markets show a good stability (low moisture content, low water activity and low microorganism load). However some microorganism indicators of fecal contamination have been detected. Furthermore, PAH content exceeded the maximal allowed limit in all investigated samples. These parameters may be considered as an important warning signal for human consumption. Therefore, important measures need to be taken to train local populations in hygienic practices as well as in controlled use of smoking technics.
ACKNOWLEDGEMENTS
- Authors are very grateful to CUD (Commission Universitaire Belge pour le Développement) for financial support, and Guy Degand for technical assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML