-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(6): 267-276
doi:10.5923/j.fph.20130306.02
Preliminary Evaluation of Antimicrobial Residue Levels in Marketed Pork and Chicken Meat in the Red River Delta Region of Vietnam
Dang Pham Kim1, 2, Guy Degand2, Caroline Douny2, Gilles Pierret3, Philippe Delahaut3, Vu Dinh Ton1, Benoît Granier4, Marie-Louise Scippo2
1Central Laboratory, Faculty of Animal Science & Aquaculture (FASA), Hanoi University of Agriculture (HUA), Gialam, Hanoi, Viet Nam
2Department of Food Sciences, Laboratory of Food Analysis, Faculty of Veterinary Medicine, CART (Centre for Analytical Research and Technology), University of Liège, bât. B43bis, Bld de Colonster 20, Sart-Tilman, B-4000 Liège, Belgium
3Laboratory of Hormonology, CER Groupe, Marloie, Belgium
4Unisensor S.A, Wandre, Liège, Belgium
Correspondence to: Dang Pham Kim, Central Laboratory, Faculty of Animal Science & Aquaculture (FASA), Hanoi University of Agriculture (HUA), Gialam, Hanoi, Viet Nam.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The presence of antibacterial in 97 pork and 83 chicken meat samples, randomly collected from 3 different representative provinces (Hanoi, Hai Duong and Thai Binh) of the Red River Delta, was determined by a screening step using in parallel 2 microbiological methods (Premi®-test and New Two Plate Test). In total, 27% of all samples displayed a positive response in at least one of both tests, from which 11 (13% of chicken samples) are chicken samples and 38 (39% of pork samples) are pork samples. The 33 samples from the Thai Binh which were screened positive were then submitted to post-screening tests specific for tetracyclines and (fluoro) quinolones (Tetrasensor® dipstick for tetracyclines and an ELISA for quinolones), two groups of antibiotics widely used in animal production in this region, and confirmed by liquid chromatography coupled to mass spectrometry. Tetracyclines and (fluoro)quinolones residues were found, using a post screening test, in 23 and 5 samples, respectively. Ten (all pork) and 4 samples (1 pork, 3 chicken) were confirmed containing tetracyclines (chlortetracycline, oxytetracycline, tetracycline, doxycycline) and (fluoro) quinolones (nalidixic acid, enrofloxacin and ciprofloxacin) respectively, from which 1 and 3 pork samples were found to contain enrofloxacin and tetracycline residues , respectively, with a concentration higher than their respective MRLs. This study shows the good performance of the proposed strategy to identify non-compliant meat samples (microbiological screening, tetracyclines and quinolones targeted post-screening and confirmation), which allows to obtain conclusive results in 82% of the cases.
Keywords: Antibiotic Residue, Chicken Meat, Pork Meat, Red River Delta Region, Vietnam
Cite this paper: Dang Pham Kim, Guy Degand, Caroline Douny, Gilles Pierret, Philippe Delahaut, Vu Dinh Ton, Benoît Granier, Marie-Louise Scippo, Preliminary Evaluation of Antimicrobial Residue Levels in Marketed Pork and Chicken Meat in the Red River Delta Region of Vietnam, Food and Public Health, Vol. 3 No. 6, 2013, pp. 267-276. doi: 10.5923/j.fph.20130306.02.
Article Outline
1. Introduction
- In Vietnam, chicken and pig, two staples products of livestock production play an important role to satisfy the increasing demand of meat for domestic markets of over 85 million inhabitants. However, the low level of hygiene in livestock, the inadequacy of husbandry zone planning and the lack of state management and development strategies result in some new problems such as environment pollution, as well as frequently occurring and uncontrolled epidemic diseases[1-3]. To overcome some of these, farmers consider antibiotics as one of the solutions to fight against livestock diseases and to improve the animal productivity. Consequently, the use of veterinary drugs and in particular antibiotics in animal production has increased in Vietnam, resulting in the fact that antibiotics are the most common registered drugs (70 percent of all veterinary drugs) used in animals[4]. The results of a survey on 628 pig and poultry farms in Binh Duong province from September 2001 to February 2002 showed that irrational use of antibiotics was recorded in 17.1% of the farms. The most used antibiotics were chloramphenicol (15.4%) and norfloxacin (10%). Furthermore, 40.1% of the farms were found not respecting the withdrawal time[5]. Another survey was performed from July 2009 to March 2010 on 270 animal production entities representing three different systems (farm household, semi-industrial and industrial) in Red River Delta (RRD). At least 48 antibiotics of more than 10 different groups were largely and arbitrary used in all pig and chicken production systems in this region[6]. This use in a unmethodical manner, without any veterinary prescription and supervision lead to the presence of residues in animal products. This issue causes bad impacts on public health and bad influences on environment[7] and therapeutic sciences[8-9] and contribute to the presence of antibiotic-resistant human pathogens in the food chain [10-14]. These alerts have caused warnings to authorities and the alarming of consumers. Since Vietnam joined the World Trade Organization, regulation of antibiotic use in animals has strengthened and certain antibiotics have been banned. Recently, Ministry of Agriculture and Rural Development (MARD) issued the updated list of drugs, antibiotics which are banned or restricted on using in animal[15].In addition, Vietnam as well as developed countries like EU, USA, has also promulgated a regulation to fix maximum residue limits (MRL) of many antimicrobial residues in animal products, control and monitoring strategy to protect consumer safety[16-21]. However, surveillance for antibiotic residues in meat reveals breaches of regulations regarding the use of antibiotics. In fact, most studies and national surveillance programs have looked at residue in animal products for export. Meanwhile, there is paucity of information on antibiotic residues in meat for domestic market. Therefore, to obtain information for an assessment of meat-borne exposure of consumers to antibiotic residue, a study of the occurrence of the residues of antibiotics widely used in pig and chicken production in RRD is necessary. The aim of this study was to assess antimicrobial residues in pork and chicken meat sold in the markets of the RRD, in Vietnam.
2. Experimental
2.1. Sampling Area
- With a population of about 19.5 million inhabitants in 15,000 km2 of superficies, the RRD region was known as an agriculturally rich area and densely populated in the north of Vietnam (1225 persons/km2, 4.8 times higher than the average population density of Vietnam). It includes the capital, Hanoi, and 10 others surrounding provinces (Fig. 1). The pig and poultry production of this region are the most developed of Vietnam (about 50% of the whole country production) with 7.0 million pigs and 66.5 million poultry in 2008[22]. Three representative provinces were selected: Hanoi (3344 km2), Hai Duong (1661 km2) and Thai Binh (1542 km2). The population density of Hanoi, Hai Duong and Thai Binh are 1943; 1030 and 1155 persons/km2, respectively. The herd of pig and poultry is the largest in Hanoi (1.2 106 pigs and 15.7 106 poultry), followed by Hai Duong (0.6 106 pigs and 6.9 106 poultry) and Thai Binh (1.0 106 pig and 7.9 106 poultry)[22].
2.2. Sample Collection
- A total of 180 meat samples (83 chicken and 97 pork samples) were randomly collected from markets of 3 districts (Dong Anh, Cam Giang and Quynh Phu) in the 3 selected provinces. Chicken and pork meat samples were taken twice a month, during 5 months from July 2009 to March 2010. At each sampling time, 3 pork and 3 chicken meat samples were taken randomly from 3 markets of each district. In the Thai Binh province, the 60 meat samples (23 chicken and 37 pork samples) were collected from July to November 2010 (just after the "blue ear" or Porcine Reproductive and Respiratory disease (PRRS) epidemic). Each sample (300 g of pork meat or breast muscle of chicken) was collected and frozen at – 80°C in separate plastic bags until the analysis.
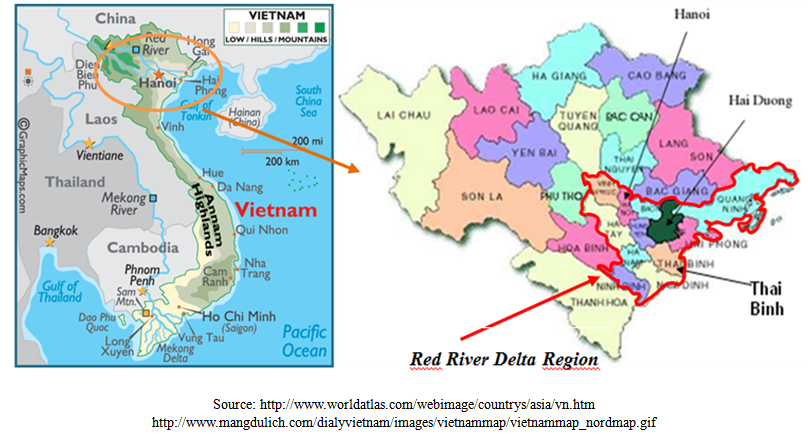 | Figure 1. Map of Red River Delta region indicating the three representative provinces where the samples were collected (Hai Duong, Thai Binh, Ha Noi) |
2.3. Strategy for Residue Analysis
- A screening step was applied by using a microbiological test to rapidly detect the samples suspected to be non-compliant. These samples were then further tested using a post-screening step in order to identify the antibiotic group responsible for the positive response at the screening stage. A confirmation analysis was performed to identify and quantify the molecule(s) possibly present in the samples giving a positive response at the post-screening stage. All samples were analyzed in parallel using two microbiological screening tests, the New Two Plate Test (NTPT) and the Premi®Test. The (fluoro) quinolones and tetracyclines are two antibiotic groups which are often found to be used in chicken and pig production in RRD[6]. For this reason, in the framework of this study, samples which displayed a positive response in one of both screening tests were analyzed using two different specific post-screening tests (the Tetrasensor® Test was used to detect tetracyclines and an ELISA to detect (fluoro)quinolones, only in samples giving a negative response after the Tetra-Sensor® analysis). Samples giving a positive response at the post-screening stage were analyzed by liquid chromatography coupled to mass spectrometry (LC-MS) (Fig. 2). The whole strategy was applied to the 60 samples from the Thai Binh province.
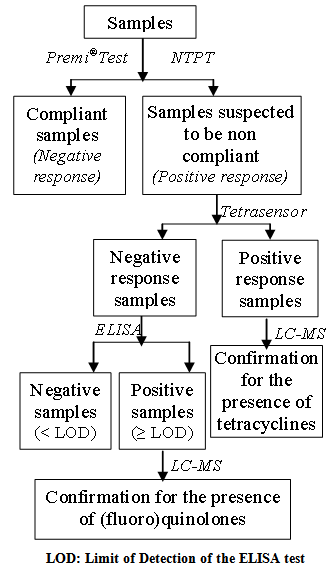 | Figure 2. Strategy of antibiotic residues analysis in pork and chicken meat marketed in the Thai Binh province, in the RRD |
2.4. New Two Plate Test (Screening test)
- The NTPT is a “home made” assay based on the growth inhibition of Bacillus subtilis. It has been recently optimized for shrimp and meat by Dang and co-workers [24-25]. Meat samples were extracted and analyzed as previously described[25].
2.5. Premi® Test (Screening step)
- Premi®Test is another kind of microbiological assay using Bacillus stearothermophilus. Test kits were purchased from DSM (DSM Food Specialities R&D, Delft, Netherlands). A multi-residue extraction step, as described by Stead and co-workers was applied to chicken and pork meat samples [23].
2.6. Group Specific Tests (Post screening steps)
- Tetrasensor® Test provided by Unisensor, S.A (Wandre, Liège, Belgium) is a receptor-based dipstick assay for a rapid screening of the presence of all the main tetracyclines in animal tissue (limit of detection ≤ 20 µg Kg-1 of tetracycline equivalents). The tetracyclines potentially present in 5 grams of sample (weighed in stomacher bags) were extracted with 15 ml of the buffer provided in the kit. After homogenizing tissue and buffer for 2 minutes with a stomacher, 2 mL of the fluid were centrifuged at 20 000 g for 1 minute. A total of 200 ml of the supernatant was transferred into the vial containing the receptor and the contents were mixed gently until the dried pellet was dissolved completely. The strip was then dipped into the vial and the whole was incubated for 10 minutes at room temperature. Meanwhile, the fluid mounts the strip and passes the two green capture lines on it, turning their color into red. The first test line binds the remaining free receptor, while the second control line binds the excess receptor. The result was read by visual inspection by comparing the color intensities of the first and the second line. The ELISA used here is a direct competitive enzyme- linked immunosorbent assay (ELISA) for the quantitative analysis of a broad range of (fluoro)quinolones residues in various matrices (using an anti-sarafloxacin antibody, a norfloxacin-peroxydase conjuguate, and a sarafloxacin calibration curve) (EIA Fluoroquinolones 2 hours E.G.3), which was provided by CER (Marloie, Belgium). The sample preparation and test procedure were performed as prescribed by the manufacturer. Five grams of homogenized sample were extracted using a simple and rapid extraction carried out with a 1:1 mixture of methanol and phosphate- buffered saline adjusted to pH 7.4. After vortexing for 30 seconds and shaking for 30 minutes, the sample was centrifuged at 4000 rpm for 10 minutes; the supernatant was transferred into a new tube. The extract (50 µl) is directly analyzed after a second centrifugation at 3000 rpm for 10 minutes, and a 10 times dilution with the dilution buffer provided with the kit. In this assay, the anti-sarafloxacin antibody is able to bind several (fluoro)quinolones, with the cross-reactivity indicated into brackets: sarafloxacin (100%), norfloxacin (105%), difloxacin (64%), ciprofloxacin (17%), pefloxacin (30%), ofloxacin (55%), flumequine (4%), cinoxacin (1%), oxolinic acid (4%), danofloxacin (88%), enrofloxacin (66%), marbofloxacin (45%), lomefloxacin (24%), enoxacin (27%) and nalidixic acid (14%). The limit of detection (LOD) is 0.5 µg Kg-1 of sarafloxacin equivalents.
2.5. Quantification of (fluoro) Quinolones (Confirmation step)
- The sample extraction procedure was adapted from the method described in the papers of Toussaint and co-workers [26-27]. Briefly, 1 g of tissue was spiked with 100 μL of lomefloxacin and 2-phenyl-4-quinoline carboxylic acid (Cincophen), both from Sigma–Aldrich (3 μg mL−1 in methanol), used as an internal standards. The extraction step was performed using 10 mL acetonitrile. A defatting step of the sample was realized by adding 3 mL hexane. The sample was vortexed, centrifuged (4000 rpm, 5 min) and then the hexane phase was discarded. The sample extract was evaporated to dryness and ammonium acetate buffer (5 mM, pH 4) was then added to obtain a final volume of 2 mL. The purification step was performed using SPE cartridges (SDB-RPS, 3 M Empore). Analytes were eluted with 4 mL of a mixture of methanol and ammonium hydroxide 1 M (75:25, v/v), the eluate was then evaporated to dryness and reconstituted with 300 μL of water/formic acid (pH 2.5). A calibration curve containing 13 (fluoro)quinolones standards (norfloxacin, ofloxacin, cinoxacin, flumequine, enoxacin, oxolinic acid, nalidixic acid, enrofloxacin, and danofloxacin mesylate from Sigma, (St Louis, MO, USA) and marbofloxacin from Vetoquinol (Belgium) was prepared with the same procedure than for the samples, using blank meat fortified at 5 different concentrations around the EU MRL[17], for each antibiotic. For antibiotics with no MRL in pork or chicken (sarafloxacin, norfloxacin, ofloxacin, cinoxacin, nalidixic acid, enoxacin), a reference concentration of 100 μg kg−1 was chosen.Identification and quantification were performed byLC–MS/MS on a 2690 Alliance separation Modules integrated autosampler, solvent delivery system and column heater coupled to a Quattro Ultima Platinum triple-quadrupole mass spectrometer (Micromass, Manchester, UK). The mass spectrometer was equipped with an electrospray ionization (ESI) interface. The LC column used was a Polaris C18A 3 μm (150 × 2.0 mm) with a Chromsep guard column SS (10 × 2.0 mm) both from Varian.The limit of quantification was 12.5 μg kg−1 for enrofloxacin and ciprofloxacin, 25 μg kg−1 for enoxacin, marbofloxacin, norfloxacin, ofloxacin, danofloxacin, cinoxacin, oxolinic acid and nalidixic acid, 37.5 μg kg−1 for sarafloxacin, 50 μg kg−1 for flumequine and 75 μg kg−1 for difloxacin.
2.6. Quantification of Tetracyclines (Confirmation step)
- Five grams of tissue were spiked with methacycline (from Dr. Ehrenstorfer, Augsburg, Germany) used as internal standard. The sample was extracted two times by blending 25 mL of succinate buffer 0.05 M pH 4 containing 20 mg of EDTA and 15 mL of hexane, to remove fat. After shaking vigorously, the samples were centrifuged; hexane was removed and the aqueous phase was transferred into a new tube. This extraction was reproduced without hexane. Both pooled supernatants were applied on a pre-conditionned SPE column (OASIS hydrophobic lipophilic-balanced (HBL), 6 mL, 200 mg, Waters Corp, Milford, MA, USA). The column was washed with 20 mL of water/methanol (95/5, v/v) and degreased with 5 mL of hexane. The tetracyclines were eluted with 5 mL methanol. The extracts were evaporated to dryness at 45°C under a flow of nitrogen. The dry residue was dissolved in 1 mL of water/methanol (70/30, v/v).The method used for quantification of tetracyclines was based on the method described by Xu et al. in 2008[28]. In short, the final separation and detection were performed by LC–MS/MS using a Quattro Ultima tandem mass spectrometer coupled to a HPLC 2690 separation module system and integrated autosampler (Micromass, Manchester, UK). The tetracyclines were detected in positive electrospray ionization (ESI) in multiple reaction monitoring (MRM) acquisition mode. A Sunfire C18 column (150 mm × 2.1 mm, 5.0 μm particle size) (Waters, Milford, MA, USA) was used for the chromatographic separation. The limit of quantification was 25 μg kg−1 for the sum of tetracyclines residues.
3. Results and Discussion
3.1. Screening Antibiotic in the Meat Samples
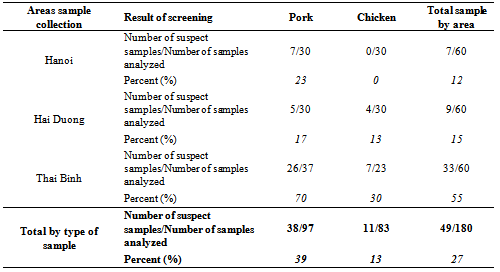
- From a total of 180 meat samples (83 chicken and 97 pork samples) collected on local markets of the RRD, 49 samples (27% of all samples) displayed a positive response in at least one of both screening tests (named “suspect samples”), from which 11 (13% of chicken samples) are chicken meat samples and 38 (39% of pork samples) are pork samples (Table 1). In the samples collected in the province of Hanoi, all the chicken meat was negative (a negative response was recorded in both screening tests) and only 7 pork samples (23% of the pork meat sampled in the province of Hanoi) were suspected to contain antibiotic residues. In Hai Duong, 17% of the pork meat samples and 13% of the chicken meat samples were screened positive. From the 49 samples screened positive (from all the 3 provinces), 33 are from the province of Thai Binh and only 16 from the other two provinces. These 16 positive results out of the 120 chicken and pork samples taken on the markets of the province of Hanoi and Hai Duong were already discussed in a previous study[25]. In the rest of this paper, we will discuss only of the 60 samples from the province of Thai Binh, to which the analytical strategy proposed in Figure 2 was applied.
|
3.2. Post-screening Analysis and Confirmation of Tetracycline and (fluoro) Quinolone Residues
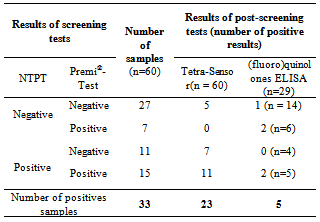
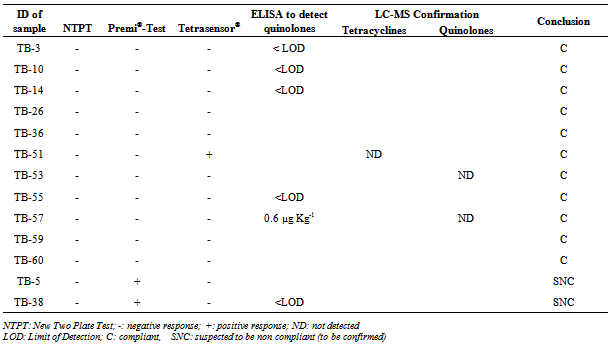
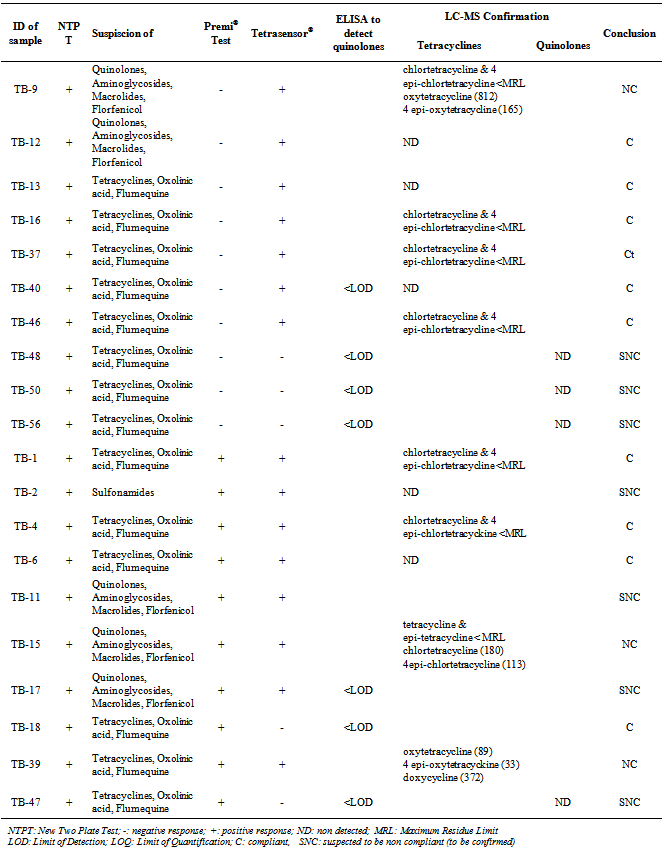
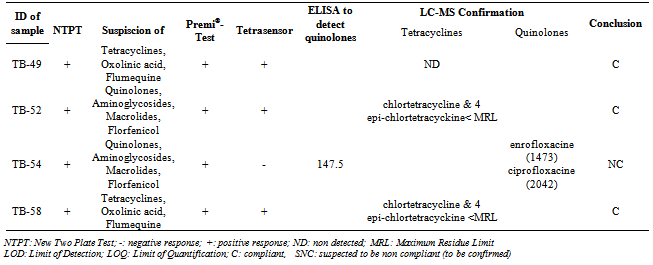
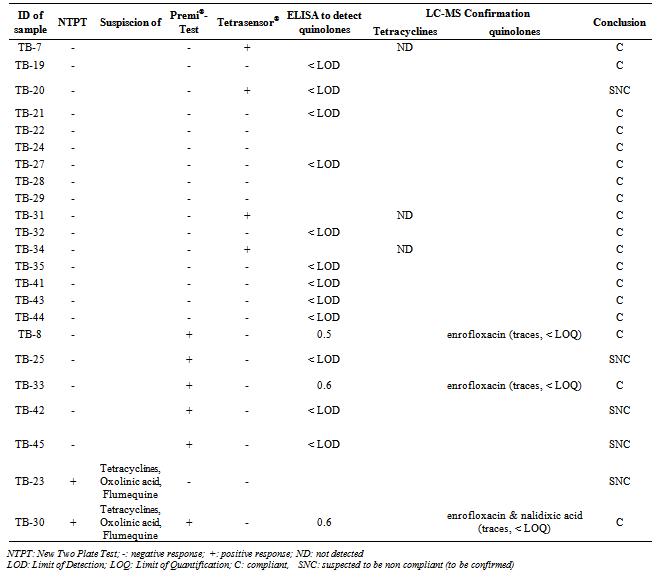
- The 60 samples taken from the Thai Binh province (presenting the higher rate of positive results) were used to evaluate the detection capacity of the screening tests used here as well as to evaluate our 3 steps analysis strategy, based on a screening, followed by a post-screening, and finally, a confirmation analysis. If we compare the results of the two microbiological screening tests (NTPT and Premi®-Test), the results (Table 2) showed that, from the 33 suspect samples, the NTPT detected 26 samples and the Premi®-Test 22 samples. Fifteen samples were detected by both tests, 11 samples displayed a positive response only with the NTPT and 7 samples only with the Premi®-test (Table 2). The interpretation results of the NTPT, according to Dang et al.[25].indicated that most of the NTPT positive samples are suspected to contain residues of both tetracycline and quinolone groups (Tables 3 and 4). These results confirm our recent survey results[6] showing that these two antibiotic groups are the most commonly used in pig and chicken production in the RRD region.
|
|
|
|
4. Conclusions
- The high proportion of pork samples containing antibiotic residues is of concern. They may cause a potential hazard to public health and particularly increase the problem of drug resistance of pathogenic bacteria. Further studies are necessary to evaluate other antibiotic residues in referred edible tissues and to estimate the risk in relation with animal product daily intakes.
ACKNOWLEDGMENTS
- This study was co-financially supported by BTC (Belgian Technical Cooperation).
References
| [1] | MARD (Ministry of Agriculture and Rural Development) (2009). Livestock development strategy to 2020, Amended and Reprinted in the first time Publishing House for Science and Technology.. |
| [2] | Ly, L.V. (2007). Livestock development in the customary process of agricultural restructuring. Hanoi, Agricultural Pub.House. |
| [3] | Ly, L.V. (2009). Sustainable livestock development in the process of industrialization. Hanoi: Faculty of Animal Science & Aquaculture (FASA), Hanoi University of Agriculture (HU). |
| [4] | An, N. Q. (2009). Report of antibiotic use in animal in Vietnam. In: Global Antibiotic Resistance Partnership- Vietnam Inaugural workshop. Hanoi: Oxford University Clinical Research Unit, National Institute of Infectious and Tropical Diseases. |
| [5] | Thuan, D. T., Tuan, N. N. & An, V. T. T. (2002). Study on antibiotic use and antibiotic residues in pig and broiler production of Binh Duong province. Veterinary Science and Technique, 10, 50-58. |
| [6] | Dang, P. K., Saegerman, C., Douny, C., Ton, V. D., Binh, D. V., Bo, H. X., Scippo, M. L. (2013). First survey on the use of antibiotics in pig and chicken production in the Red River Delta Region of Vietnam. Submitted to Food and Public Health. |
| [7] | Cabello, F. C. (2006). Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environmental Microbiology, 8, 1137-1144. |
| [8] | WHO (1997). The Medical Impact of Antimicrobial Use in Food Animals. Report of a WHO Meeting., Berlin, Germany, 13-17 October 1997.R. Weisshaar, B. Gutsche, "Formation of acrylamide in heated potato products - model experiments pointing to asparagine as precursor", Deutsche Lebensmittel- Rundschau, vol. 98, pp.397-400, 2002. |
| [9] | WHO (2001). Monitoring antimicrobial usage in food animals for the protection of human health. Report of a WHO consultation Oslo, Norway 10-13 September. |
| [10] | Adam, D. (2002). Global antibiotic resistance in Streptococcus pneumoniae. J Antimicrob Chemother, 50 Suppl, 1-5. |
| [11] | Molbak, K. (2004). Spead of resistant bacteria and resistance genes from animals to humans – The public health consequences. J. Vet. Med., B 51, 364-369. |
| [12] | Sarmah, A. K., Meyer, M. T. & Boxall, A. B. A. (2006). A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere, 65, 725-759. |
| [13] | Duong, H. A., Pham, N. H., Nguyen, H. T., Hoang, T. T., Pham, H. V., Pham, A.M., Berg, M., Giger, W. & Alder, A. C. (2008). Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam. Chemosphere, 72, 968-73. |
| [14] | Wang, H. H., Manuzon, M., Lehman, M., Wan, K., Luo, H. L., Wittum, T. E., Yousef, A. & Bakaletz, L. O. (2006). Food commensal microbes as a potentially important avenue in transmitting antibiotic resistance genes. Fems Microbiology Letters, 254, 226-231. |
| [15] | MARD (Ministry of Agriculture and Rural Development) (2010). Ministerial Circular No. 20/2010/TT-BNNPTNT of 02 avril 2010 on supplement and amend the Circular No. 15/2009/TT-BNN dated 17/3/2009 of the Minister of MARD promulgating the list of drugs, chemicals and antibiotics banned or restricted use. |
| [16] | FAO/WHO (2004). Residues of some veterinary drugs in animals and foods. Sixty-second report of the Joint FAO/WHO Export Committee on Food Additives. WHO Technical Report Series, FAO FNP 41/16. Rome, 4–12 February. |
| [17] | EU (2010). Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Official Journal, L15, 1-72. |
| [18] | EC (1996). Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residue thereof in live animals and animal products and repealing Directive 85/358/EEC and 86/469/EEC and Decision 89/187/EEC and 91/664/EEC. Official Journal, L125, 10-32. |
| [19] | FSIS (2006). Red Book. From:http://www.fsis.usda.gov/PDF/2006_red_book.pdf. Retrieved March 05, 2011. |
| [20] | FSIS (2009). Directive 10220.3. From:http://www.fsis.usda.gov/OPPDE/rdad/FSISDirectives/10220-3.pdf. Retrieved March 05, 2011. |
| [21] | VMOH (2007). (Vietnamese Ministry of Health). Decision No. 46/2007/QĐ-BYT of 19th December 2007, promulgating regulations on the maximum residue limits of biological and chemical substances in foodstuffs (In Vietnamese). |
| [22] | GSO (General Statistics Office), 2009. Statistical data. Available from:http://www.gso.gov.vn/default.aspx?tabid=426&idmid=3. Retrieved April 16, 2011. |
| [23] | Stead, S., Sharman, M., Tarbin, J. A., Gibson, E., Richmond, S., Stark, J. & Geijp, E. (2004). Meeting maximum residue limits: an improved screening technique for the rapid detection of antimicrobial residues in animal food products. Food Additives and Contaminants, 21, 216-221. |
| [24] | Dang, P.K., Degand, G., Danyi; S., Pierret; G., Delahaut; P., Dinh; T.V., Maghuin-Rogister, G., Scippo, M.L. (2010). Validation of a two-plate microbiological method for screening antibiotic residues in shrimp tissue. Analytica Chimica Acta, 672, 30-39 |
| [25] | Dang, P. K., Degand, G., Douny, C., Ton, V. D.,Maghuin-Rosister, G. & Scippo, M. L. (2011). Optimization of a new two-plate screening method for the detection of antibiotic residues in meat. International Journal of Food Science & Technology, 46, 2070–2076 |
| [26] | Toussaint, B., Bordin, G., Janosi, A. & Rodriguez, A. R. (2002). Validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of 11 (fluoro)quinolone antibiotics in swine kidney. Journal of Chromatography A, 976, 195-206. |
| [27] | Toussaint, B., Chedin, M., Bordin, G. & Rodriguez, A. R. (2005). Determination of (fluoro)quinolone antibiotic residues in pig kidney using liquid chromatography-tandem mass spectrometry I. Laboratory-validated method. Journal of Chromatography A, 1088, 32-39. |
| [28] | Xu, J. Z., Ding, T., Wu, B., Yang, W. Q., Zhang, X. Y., Liu, Y., Shen, C. Y. & Jiang, Y. (2008). Analysis of tetracycline residues in royal jelly by liquid chromatography-tandem mass spectrometry. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 868, 42-48. |
| [29] | Goodman, J.J. (1985). Fermentation and Mutational Development of the Tetracyclines. Handbook of Experimental Pharmacology, 78, 5-57. |
| [30] | DAH (Department of Animal Health), Update on Highly Pathogenic Avian Influenza, Foot and Mouth disease and Porcine reproductive and respiratory syndrome situation, (2010) Available fromhttp://www.cucthuy.gov.vn/index.php?option=com_content&task=category§ionid=1&id=19&Itemid=64, 2010. Retrieved 04/3/2011 |
| [31] | Canavan, A. Capacity Building for Veterinary Drug Residue Monitoring Programmes in Developing Countries. Joint FAO/WHO Workshop on Residues of Veterinary Drugs without ADI/MRL-Bangkok; 2004. Available from:http://www.fao.org/docrep/008/y5723e/y5723e0g.htm Retrieved 10/03/2011 |
| [32] | EFSA (European Food Safety Authority), EFSA in focus AlimentAtion. Édition 07 - JUILLET 2010 Available from http://www.efsa.europa.eu/fr/focusfood/docs/food7fr.pdf Retrieved 20/12/2010. |
| [33] | Al-Mazeedi, H. M., Abbas, A. B., Alomirah, H. F., Al-Jouhar, W. Y., Al-Mufty, S. A., Ezzelregal, M. M. & Al-Owaish, R. A. Screening for tetracycline residues in food products of animal origin in the State of Kuwait using Charm II radio-immunoassay and LC/MS/MS methods. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment, 2010. 27, 291-301. |
| [34] | Salama, N. A., Abou-Raya, S. H., Shalaby, A. R., Emam, W. H. & Mehaya, F. M. Incidence of tetracycline residues in chicken meat and liver retailed to consumers. Food Additives & Contaminants Part B-Surveillance, 2011. 4, 88-93. |
| [35] | Thuat, D. T., Tuan, N. N., An, V. T. T., Hien, L. T., Lam, V. B., Ninh, K. T. Study on antibiotic use and antibiotic residues in pork and broiler meat of Binh Duong province”, 2002, Journal of Veterinary science and Technology, X (1), 50-57. |
| [36] | Nhiem, D.V., Peter, P., Witaya, S., Frans, J. M. S., Moses, N.K., Maximilian, P.O. B., Karl, H.Z., Ngan, P.H. Preliminary Analysis of Tetracycline Residues in Marketed Pork in Hanoi, Vietnam. Annals of the New York Academy of Sciences, 2006, 1081, 534–542. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML