-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(4): 195-214
doi:10.5923/j.fph.20130304.04
ObtainingAntioxidants from Botanic Matrices Applying Novel Extraction Techniques
Moyses N. Moraes, Giovani L. Zabot, Juliana M. Prado, M. Angela A. Meireles
School of Food Engineering, University of Campinas, UNICAMP, R. Monteiro Lobato, 80, CEP: 13083-862, Campinas, SP/Brazil
Correspondence to: M. Angela A. Meireles, School of Food Engineering, University of Campinas, UNICAMP, R. Monteiro Lobato, 80, CEP: 13083-862, Campinas, SP/Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Botanic matrices are abundant sources of antioxidants which have the capacity to avoid the lipid oxidation of food and present remarkable health benefits. The natural antioxidants might be obtained applying many extraction techniques. Satisfactory results of obtaining extracts with antioxidant properties and high yields using modern extraction techniques are shown by recent studies. The selection of the suitable technique depends on the desired class of substances to be extracted. In this overview, the advances reached in scientific researches involving natural antioxidants are presented. The advantages and potential applications of four novel extraction techniques: Supercritical Fluid Extraction, Pressurized Liquid Extraction, Microwave Assisted Extraction and Ultrasound Assisted Extraction are discussed, considering the characteristics of the target compounds. These techniques reduce the solvent consumption and abridge the extraction time. Consequently, the process productivity is increased.
Keywords: Antioxidants, Bioactive Compounds, Flavonoids, Phenols, Phytochemicals, Pressurized Liquid Extraction, Supercritical Fluids
Cite this paper: Moyses N. Moraes, Giovani L. Zabot, Juliana M. Prado, M. Angela A. Meireles, ObtainingAntioxidants from Botanic Matrices Applying Novel Extraction Techniques, Food and Public Health, Vol. 3 No. 4, 2013, pp. 195-214. doi: 10.5923/j.fph.20130304.04.
Article Outline
1. Introduction
- The interest in the prevention of chronic diseases, such as cancer, has led to modifications in the nutritional composition of foods in the last years. These modified foods are classified as functional foods because they contain, besides the basic nutrition, components that provide health benefits[1]. The continued ingestion of food supplemented with antioxidant substances cause inhibitory effects on the proliferation of carcinogenic cells in human beings[2-4]. These effects appear because the antioxidant substances are able to perform some functions, as free radical scavenging, peroxide decomposition, suppression of singlet oxygen, enzymatic inhibition[1] and increasing the levels of endogenous defences[5].In general, there are two categories of antioxidants: natural and synthetic. The natural antioxidants comprise a wide variety of substances found in the nature, such as polyphenols (flavonoids and phenolic acids), terpenoids and vitamins E and C. The phenolic compounds present high antioxidant capacity in biologic and food systems, especially the flavonoids group[6].The identification of the chemical composition of extractsfrom several botanic matrices that present agents with potential antioxidant properties has been the focus of many studies in food and health fields. Some antioxidants are thermolabile, sensitive to light and they interact with polar and nonpolar solvents by different mechanisms. These characteristics can change the extraction yield and the quality of the extract recovered. The objective of this overview is to report the advantages associated with some modern extraction techniques for obtaining naturalantioxidants. The extraction technique more suitable for the extraction of a given target group is indicated.
2. Natural Antioxidants and Their Health Benefits
- In this section, the beneficial health effects exercised by phytochemical compounds with antioxidant properties are briefly gathered. Epidemiologic studies showingexperimental evidences of the relationship between better health and diets rich in food containing these phytochemicals are referenced. Emphasis is given to recent studies which show the current results of ingesting natural antioxidants as polyphenols, terpenoids and vitamin E.
2.1. Polyphenols
- Polyphenols are defined as substances which contain an aromatic ring attached to one or more hydroxyls, including their functional derivatives[7]. A wide variety of phenolic derivatives with antioxidant capacity is found in botanic matrices, including simple phenols, benzoic and cinnamic acids derivatives and flavonoids.
2.1.1. Flavonoids
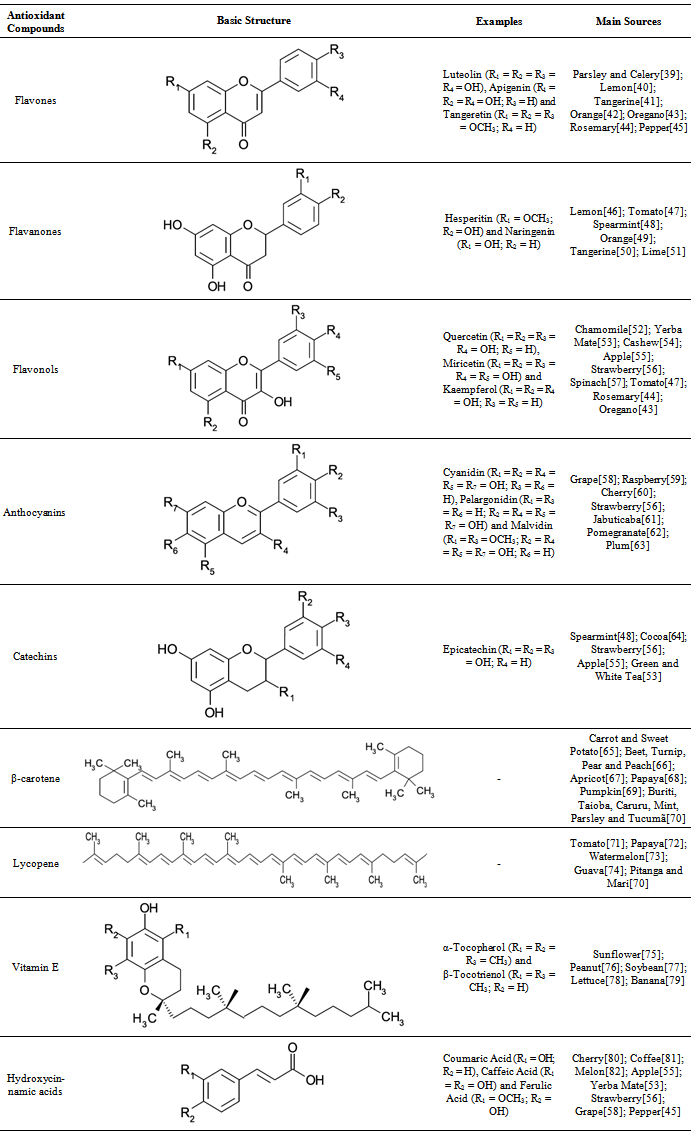
- Flavonoids are secondary metabolites of low molecular massproduced by plants, which belong to the class of phenolic compounds and present high antioxidant activity[7]. They act as antioxidants because they have many active sites to scavenge free radicals[8]. The flavonoids are divided into groups according to their chemical structures, as flavones and isoflavones, flavanones, flavonols, flavanols and anthocyanins (Table 1).Living organisms have an oxidation-reduction system necessary to keep the level of generated free radicalsconstant. The formation of free radicals in higher levels than the ideal induces cellular oxidative stress that leads to lipid peroxidation of the cellular membrane, which can cause degenerative diseases and aging[9]. Therefore, scientific investigations search for solutions to avoid the cellular oxidation by the supplementation of antioxidant compounds in food. There are evidence that flavanols, flavanones and anthocyanidins have beneficial effects to the memory, perception and neurodegeneration[10].Hemodialysis patients face an elevated risk of cancer, ascribed in part to increased oxidative stress. Anthocyanins, present at high amounts in red fruits, show a good efficacy on the reduction of the oxidative damage in these individuals through the decrease on the risks of DNA oxidation and on the lipid and protein peroxidation[11].Saponarin, an antioxidant belonging to the flavones group and that was recently found in barley leaves, inhibited the malonaldehyde formation. In a normal reaction,malonaldehyde is formed from oxidized lipids on the skin surface by ultraviolet irradiation[12]. Naringin, a dietetic flavanone, is an effective antioxidant for the prevention of oxidative stress and for the protection against liver carcinogenesis in rats[13]. In human beings, phenolic extracts obtained from apple and grape containing flavonoids showed potential protection of lung cells exposed to the oxidative stress[14].Proanthocyanidins extracted from blueberry (Vaccinium angustifolium) are able to reduce the cognitive function loss by the protection against the deficient Ca2+ recovery and moderate oxidative / inflammatory stress signalling[15]. Flavonoids extracted from fennel (Foeniculum vulgare) present antitumoral effect by modulating the lipidperoxidation[16]. Flavonoids extracted from carob (Ceratonia siliqua), mostly the miricetin, caused biochemical changesin rats physiologic system, suggesting protection of liver and renal cells by the capacity of free radicals scavenging[17].Flavanols extracted from lychee (Litchi chinensis) were supplemented on the diet of 20 healthy male long-distance runners. The decrease of the cellular oxidative stress and the reduction of the tissue damage caused by high-intensity exercise training were observed[18].
2.1.2. Non-flavonoids
- The non-flavonoids compounds are phenolic acids which present a functional carboxyl group, divided into benzoic and cinnamic acids derivatives[7]. Hydroxycinnamic acids are present in many foods, such as coffee, yerba mate, apple and plum (Table 1)[19]. The phenolic acids presentimportant biologic and pharmacologic properties, particularly on cancer prevention[20].Caffeic acid, a hydroxycinnamic acid found in high concentrations in fruits and coffee beans, induces the apoptosis of human breast cancer cells[21]. The use of this phytochemical for the protection against disturbances of the antioxidant defense system has been tested as the possible mechanism whereby botanic compounds slow down the skin aging process. Pretreatment of skin cells with caffeic acid prior UVA (ultraviolet A) irradiation inhibits cytotoxicity, induction of metalloprotease-1 (enzyme responsible for the damage caused on collagen) and free radicalsgeneration[22].Ferulic acid is a powerful phenolic antioxidant and photo-protector obtained from plants such as corn, rice, tomato, peanut, apple, orange and pineapple. Ferulic acid decreases the absorption of UVB (ultraviolet B) radiation on human epidermis and inhibits the formation of tumors, because this compound blocks the secretion of cytokines generated after the skin is exposed to the UVB radiation[23].
2.2. Terpenoids
- Terpenoids are classified according to the number of carbon atoms in their chain.β-carotene and lycopene are tetraterpenoids (carotenoids formed by 40 carbons)[24]. The main vegetal sources of carotenoids and their molecular structures are listed in Table 1.Scientific evidences link the antioxidant properties of carotenoids with their beneficial effect against chronic diseases. Annatto (Bixa orellana) extract, constituted by carotenoids with bixin as major compound, was identified as a potential therapeutic agent for modulation of the equilibrium of reactive oxygen and nitric oxide species, two substances that induce diabetes[25]. Annatto constituents were also studied as toxic agents against a wide variety of tumor cells. Cis-bixin has the capacity of inhibiting the enzymes associated with the oxidative stress[26].Experimental assays point out that lycopene can protect the organism against damages caused by the exposure to tobacco[27],[28], and it is beneficial on the treatment of acute and chronic pancreatitis by reducing intracellular free radicals[29]. β-carotene can help the prevention of prostate cancer[30]and gastric carcinoma[31].
2.3. Vitamin E
- The supplementation with vitamin E in the diet of180healthy elderly people during 4 months apparently alleviates the oxidative stress by improving the erythrocyte membrane fluidity and by reducing the erythrocyte hemolysis[32]. Vitamin E tested in rats inhibited the formation of oxygen reactive species, decreased the level of lipid peroxide, increased the levels of glutathione and lipid peroxidation enzymes and presented the capacity to prevent the mitochondrial apoptosis[33].Vitamin E identified in garlic extract was tested against the cellular oxidative stress in rats. This phytochemical helped on the protection of the liver structural integrity due to free radicals scavenging capacity[34]. Other beneficial health effects provided by vitamin E include cardiovascular diseases prevention[35], chemo preventive actions against skin cancer[36] and induction of apoptosis of carcinogenic pancreatic cells[37]. All forms of vitamin E are able to induce antioxidant effects and to protect food and biologic membranes against the lipid peroxidation[38].
|
3. Modern Techniques for Extraction of Antioxidants
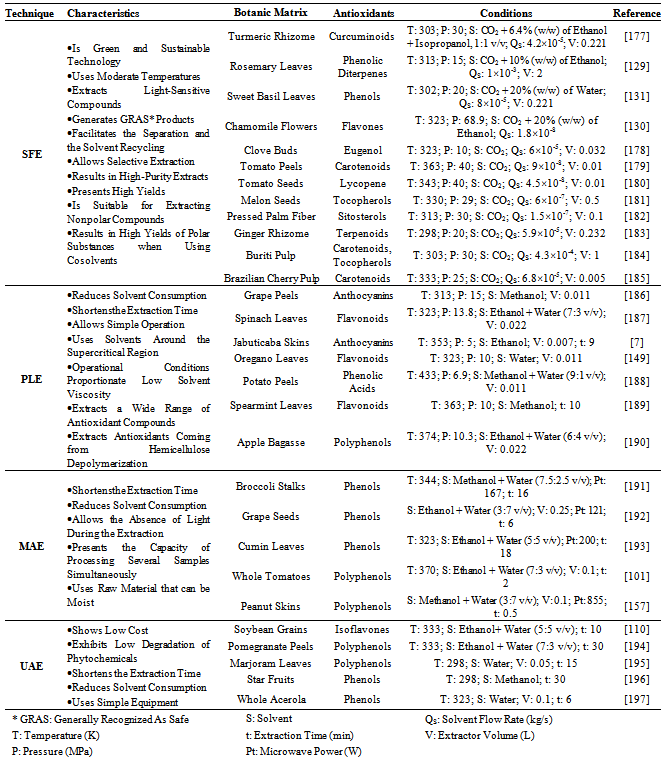
- Antioxidants are substances that, in low concentrations, inhibit or prevent the oxidation of other substances[83]. Many foods still contain synthetic antioxidants in their formulations, as Butylated Hydroxytoluene (BHT) and Butylated Hydroxyanisole (BHA), because they are thermally stable and of low cost. However, experimental investigations show that BHT and BHA are carcinogenic and cytotoxic above 500 ppm[84]. The maximum recommended BHT daily intake is 0.125 mg/kg of body mass and the maximum recommended BHA daily intake is 0.5 mg/kg of body mass. In the European Union the use of BHT and BHA in food prepared for babies and young children is not allowed[85].Due to these issues, natural antioxidants allowing for the substitution of synthetic antioxidants are the target of many studies[86-91]. Boo et al[92] demonstrated high antioxidant activities of natural pigments found in onion (Allium cepa L.), red cabbage (Brassica oleracea L.), mulberry (Morus alba L.), purple sweet potato (Ipomoea batatas L.), yellow paprika (Capsicum annuum L.), red beet (Beta vulgaris L.) and grape (Vitis vinifera L.).Botanic matrices are abundant sources of nutraceutical compounds. The natural antioxidants, belonging to the GRAS (Generally Recognized As Safe) group of FDA (Food and Drug Administration), are extracted from herbs or plants and are commonly phenolic compounds that present health benefits, as the prevention of diabetes, cancer, hypertension, asthma and infections[93],[94].The separation or the isolation of the target compounds from their original matrix is the method used to obtain these antioxidant substances. Conventional extraction techniques (steam distillation and Soxhlet extraction, for instance) possess some drawbacks due to the use of high temperatures and/or high amounts of organic solvents; another limitation is that the steam distillation process can be used only to obtain volatile oils (mostly terpenes). These conventional techniques are being substituted by novel techniques, as supercritical fluid extraction (SFE)[95-98]; pressurized liquid extraction (PLE)[90],[99],[100]; microwave assisted extraction (MAE)[101-103]; and ultrasound assisted extraction (UAE)[104],[105]. The choice of the suitable technique depends on: the desired class of compounds to be extracted; the structural characteristics of the botanic matrix (fruits, stems, seeds, leaves, root, flowers, etc.); the quality and yield required for the extract; the process conditions (temperature, pressure, etc.) and the economic feasibility for scaling up the process.For instance, SFE using pure CO2 is more appropriate for extracting nonpolar compounds as terpenoids, tocopherols and sitosterols[106], while PLE is more appropriate for extracting polar antioxidants as the phenolic compounds: anthocyanins[107] and flavonols[108]] using solvents with high polarity. MAE is an extraction technique indicated when the botanic matrix contains large amounts of water, because the water is responsible for the absorption of the energy generated by the microwaves. This energy disrupts the cells and facilitates the release of chemical constituents[109]. The UAE technique is also suitable for obtaining antioxidants. The characteristic of UAE is the reduced solvent consumption[110], the possibility of processing several samples in the same equipment and the short extraction time[109].The solvent selection for the extraction is based on some factors, as: physicochemical properties, availability, cost and toxicity. The choice of the ideal solvent should consider its selectivity, as well the solute solvating capacity, interfacial tension, viscosity, stability and reactivity[109].
3.1. Historical Aspects
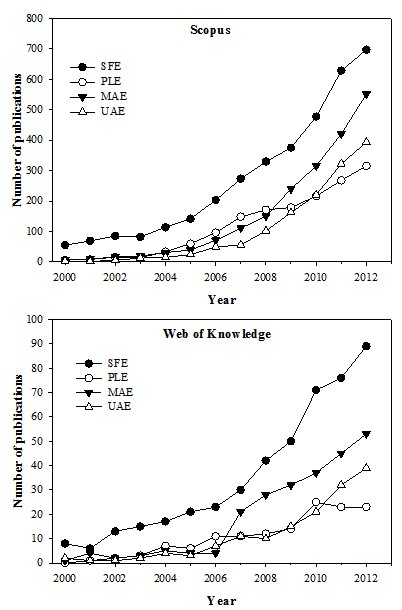
- The application of supercritical technology in obtaining bioactive compounds has evolved over the past decade. However, the divulgation of the investigations related to this area in patent form started in the early of 1970 when the first patent comprising a process for recovering caffeine from green coffee using carbon dioxide in supercritical conditions was registered by Zosel[111]. In 1981, another patent was published dealing with the decaffeination of coffee[112]. From that date to now, over than 300 patents were registered and are available at “Web of Knowledge” database. One of these recent patents comprises a useful method for preparing carotenoid microcapsules with a controllable isomeric ratio applying a supercritical fluid at high temperatures[113]. In the same way, another invention utilizes olive by-products for the isolation and separation of tocopherols with supercritical fluids[114]. In 1997, a inovative study was published dealing with the effects of ultrasound on mass transfer in SFE[115]. This coupled system has been investigated currently in the extraction of lutein esters from marigold[116] and of oil from adlay seed[117].The number of scientific investigations published after the year 2000 comprising modern extraction techniques for obtaining antioxidants has significantly risen by 2012. Figure 1 shows this tendency, whereby searches in “Scopus” and “Web of Knowledge” database inserting the terms “SFE and antioxidant” returned together more than 780 documents in the year 2012, while less than 80 documents covering the same subject were published in the year 2000. The same search procedures were used for PLE, MAE and UAE. These novel techniques also present an important evolution in the scientific scenario, mainly in the last five years where the number of publications related to MAE and UAE in Scopus, for instance,increased from 150 and 102 to 551 and 393, respectively. The participations of SFE, PLE, MAE and UAE in obtaining natural antioxidants referent to the overall techniquesin 2003 have been 5.9%, 1.0%, 1.4% and 0.9%, respectively. In 2012, these relative participations have increased to 9.5%, 4.3%, 7.5% and 5.4%, respectively. Therefore, the contribution of both techniques in 2003 has been 9.2%, while in 2012 it has been 26.7%, almost 3 times higher than ten years ago.
3.2. Supercritical Fluid Extraction (SFE)
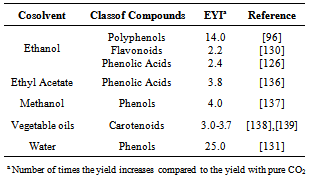
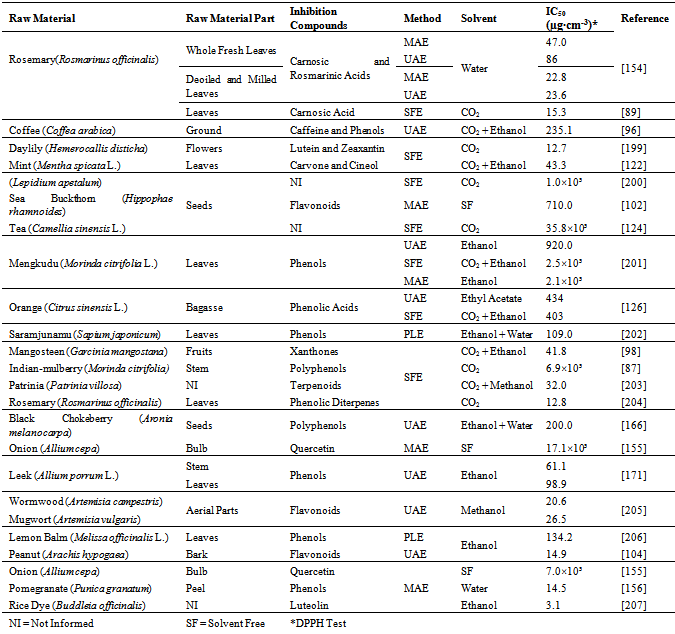
- Extraction of bioactive compounds with conventional solvents is characterized by low selectivity and may require high temperature[118]. Because of these limitations, SFE has some characteristics that justify its use for obtaining natural antioxidants. CO2, the solvent mostly used in SFE, presents critical temperature of only 304 K, which allowsits use for the extraction of thermo sensitive (thermolabile) compounds.Several phytochemicals show high solubility in CO2around supercritical conditions (304 K/ 7.4 MPa).Bioactive compounds extracted from botanic matrices by SFE technique present a pronounced reproduction of the sensory characteristics of the raw material when compared to conventional techniques. The thermal degradation and the decomposition of thermolabile substances are strongly reduced, since the SFE procedure occurs at low temperatures and in the absence of oxygen and light. This feature is especially useful in the extraction of antioxidants, because it guarantees the conservation of their functionalproperties[119]. Moreover, SFE is more selective than the conventional extraction techniques, and it is suitable for obtaining solvent-free products[118].Extraction of antioxidants with supercritical CO2 requires the use ofhigh pressures. At this condition, the co-extraction of other undesirable compounds, as waxes and oleoresins, might happen. When the co-extraction of these compounds cannot be avoided, several separator vessels can be displayed in series, operating at different temperature and pressure conditions, to fractionate the extract[120].Belonging to the Lamiaceae family, rosemary(Rosmarinus officinalis) is a plant with powerful antioxidant agents. Carnosic acid (CA) and carnosol are the major phenolic diterpenes present in rosemary extracts obtained by SFE, as shown by Kuo et al.[121]. The CA content obtained in the referred study was approximately 110 mg/g extract, resulting in IC50 of 7.47 µg/cm3. The IC50 is the concentration of extract or active compound needed to inhibit 50% of oxidation of a defined substance, which can be determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) test. An IC50 of 7.47 µg/cm3is very attractive, because low concentrations of CA present significant effects on the free radicals scavenging. At the concentration of 80 µg/cm3, CA presented inhibition of84.1% of the lipid peroxidation, while the synthetic BHT antioxidant inhibited 80.8% of the lipid peroxidation.The results obtained by Kuo et al.[121] corroborate the studies carried out by Vicente et al.[89] using rosemary leaves; the authors obtained high CA content in the extract in 1 h of extraction by SFE. The antioxidant activity of the extract increased with the extraction time because the volatile oil is depleted from the vegetal matrix at the beginning of the process, and the phenolic compounds, which present higher antioxidant activity, are only later extracted.Antioxidants are compounds usually sensitive to light and heat. The antioxidants obtained by SFE have the advantage of being processed under the absence of light and at moderate temperatures. Furthermore, they are easilyseparated from the solvent and they hardly suffer undesirable oxidation reactions. Recently, natural antioxidants with high activity were obtained by SFE from mint (Mentha spicata L.)leaves[122],[123], coffee (Coffea arabica)grounds[96], green tea (Camellia sinensis) leaves[124], grape (Vitis vinifera L.) seeds[97], thyme (Thymus vulgaris)flowers[125], guava (Psidium guajava L.) seeds[86], orange (Citrus sinensis L.) bagasse[126] and rosemary (Rosmarinus officinalis) leaves[127].The interest in SFE has been increasing in the last years, which is shown by the several studies found in literature dealing with this topic (Figure 1). One of SFE features is that the raw material must be dried prior to the extraction with supercritical CO2. The water decreases the efficiency of this technique by limiting the contact between the CO2 and nonpolar solutes[120]. The water present in the solid material may also compete with CO2 to dissolve the solute, which affects the mass transfer rate. Considering these aspects, the drying of the raw material at ideal conditions, without causing degradation of the bioactive compounds, is required[109].Supercritical CO2 is a solvent appropriate to extract nonpolar solutes. Compounds presenting high molecular mass, as flavonoids, are poorly soluble in pure CO2. In such case, the addition of a polar cosolvent to the CO2, to form a mixture with it in ideal proportions, improves the solubility of polar organic compounds. Thus, the mixture CO2 + cosolvent can increase the mass transfer rate. The solubility improvement in the supercritical region is associated with molecular interactions, mostly hydrogen bonds[128].The extraction of polyphenolic antioxidants by SFE with cosolvents was studied by various authors. Phenolic diterpenes were obtained from rosemary using 10% (w/w) of ethanol[129] and flavones were extracted from chamomile (Matricaria recutita) using 20% (w/w) of ethanol[130]. Water was employed as a cosolvent for extracting phenols from sweet basil (Ocimum basilicum)[131] and antioxidant compounds from sunflower (Helianthus annuus)[132] at proportions of 20% (w/w) and 5% (w/w), respectively.Ethanol and water are the most appropriatesolvents to be applied in the food industry. Ethanol is widely used to increase the efficiency of the extraction of phenolic acids and flavonoids, and is easily removed from the final product by distillation[120],[133]. The cosolvents commonly used to extract antioxidant compounds are listed in Table 2.
|
3.3. Pressurized Liquid Extraction (PLE)
- The PLE technique is an alternative that has been recently used to obtain bioactive compounds; it uses an aqueous or organic solvent at high pressure and/or temperature by circulating the solvent through the sample. High pressure is not the most important feature in PLE process. In fact, most often the purpose of raising the pressure is to keep the solvent in the liquid phase. Designations for PLE can be found in literature, as accelerated solvent extraction (ASE), pressurized hot solvent extraction (PHSE), pressurized water extraction (PWE), high pressure solvent extraction (HPSE) and subcritical solvent extraction (SSE), among others. Antioxidants can be obtained using solvents at temperatures above their boiling point. For these reasons, a generic term is used: “superheated solvent extraction” (SHSE)[140]. Liquid carbon dioxide cannot be used in this case because its critical temperature is low (at about 304 K) compared to the temperatures used in PLE. So, at pressures and temperatures above the critical point of CO2 the process is called SFE.Flavonoids, catechins, anthocyanins, flavanones, andflavones are some of the phenolic compounds which were obtained using PLE[107],[108],[141-144]. King andGrabiel[145], in their patent, demonstrated the potential of PLE technique for extracting polyphenols from fruits and vegetables wastes. Also, a method for extraction of phenols from grape skins by ASE using ethanol-water mixtures is found in literature[146].In PLE, high temperature is usually attractive, because it improves the extraction yield. The increase in temperature modifies the solvent dielectric constant and the solute solubility in the solvent[109]. Studies show that polyphenols extracted at temperatures above 363 K are unstable and can suffer pronounced thermal degradation, although the quantity of antioxidants extracted is high at elevated temperatures[107],[147],[148].Comparative assays using PLE and conventional extraction techniques were carried out for obtaining the three major flavones (hesperitin, nobiletin and tangeretin) present in tangerine peels (Citrus reticulata). The flavones were efficiently extracted by PLE, reaching higher yields than the conventional methods. Additionally, the extraction time was lower for PLE[141].The recovery of phenolic compounds from oregano leaves (Origanum vulgare) by PLE was tested by Miron et al.[149]. The operational conditions used in the experimental assays were temperatures of 323 K, 373 K, 423 K and 473 K, and different proportions of ethanol/water as solvents. The extracts obtained using PLE with 100% of water in batch mode applying an 11 cm3 extractor at 323 K and 10 MPa presented the highest amount of phenols and the highest antioxidant activity. Under these conditions, the total phenols content was 184.9 mg GAE/g extract, where GAE means “gallic acid equivalents”. The antioxidant activity was established as the amount of extract necessary to reduce the DPPH concentration in 50%, resulting in IC50 of only 6.98 µg/cm3. When pure ethanol was used as solvent at temperature of 373 K, the total phenols content was only 102.2 mg GAE/g extract and the IC50 was 11.5 µg/cm3. These results suggest that the solvent and the temperature influenced both the extract yield and its quality[149].Recovery of anthocyanins and phenolic compounds from jabuticaba (Myrciaria cauliflora) was studied applying PLE and Low-Pressure Solvent Extraction (LPSE). Similar yields were obtained using both techniques. However, the PLE technique was attractive because it resulted in a rapid process (≈ 9 min) and it allowed low solvent consumption. The content of anthocyanins and total phenols in PLE extract were 2.15 and 1.66 times higher, respectively, than their content in LPSE extract. Moreover, PLE extract resulted in cost of manufacturing 40 times lower than LPSE extract due to the short processing time[150].PLE technique is usually appropriate for obtaining antioxidants from lignocellulosic materials. Some of the effects achieved under temperatures of hydrothermal treatment (for instance above 493 K) are listed as[120]:i) solubilizationof acid-solublelignin;ii) hydrolytic depolymerization of hemicellulose into compounds of highmolecular mass (soluble fibers);iii) extractionof lipophilic compounds;iv) extraction of lignans;v) extraction of non-saccharides as terpenes, fatty acids and monomeric phenols.The phenolic compound vanillin was the major component with antioxidant activity found in barley husks subjected to non-isothermal auto-hydrolysis in aqueous medium[151]. The solubilized portion obtained in the auto-hydrolysis of pine (Pinus radiata) using several tests (DPPH radicalscavenging, hydroxyl radical scavenging, Trolox equivalent antioxidantcapacity, b-carotene bleaching and reducing power) presented specific antioxidant activity 40 times higher than BHT, 25 times higher than α-tocopherol, 8 times higher than caffeic acid, 3.5 times higher than BHA and 3 times higher than gallic acid[152].PLE differs from conventional techniques because PLE uses high temperature and pressure in extractive process, which may be conducted in semi-continuous (dynamic) or batch (static) modes. A wide range of temperature might be applied; it usually varies between 293 K and 473 K. The pressure commonly used varies between 3 MPa and 20 MPa[153]. Therefore, PLE is a suitable technique for extracting several solutes, both polar and nonpolar. PLE has been used as an alternative for obtaining antioxidant substances with high molecular mass derived from the hemicellulose fragmentation[120].
3.4. Microwave Assisted Extraction (MAE)
- MAE is another innovative technique that has beenpresenting special interest. Microwaves consist of nonionizing electromagnetic energy with a frequency from 0.3 GHz to 300 GHz that is applied directly to the raw material. They transmit energy which penetrates into the biologic matrix and interacts with polar molecules, mostly water, generating heat; the heat expands and disrupts the vegetal cell, favoring the extraction of intracellular phytochemical compounds. MAE is a technique frequently used for extractigthermolabile compounds[109].Extraction of phenolic compounds from cherry (Prunus cerasus)pulp was performed using the MAE method in batch mode by Simsek et al.[103]. Epicatechin (flavanol) was the major phenolic compound extracted, and its concentration was higher when using the MAE technique than when using the conventional technique. The antioxidant efficiency of MAE extract was 28.32 mg DPPH/g sample[103]. Antioxidants present in deoiled rosemary (Rosmarinus officinalis) leaves were extracted by MAE using ethanol and water as solvents. Carnosic acid and carnosol were responsible for most of the antioxidant activity found in the extract. The IC50 values using water and ethanol as solvents were 22.8 µg/cm3 and 41.0 µg/cm3, respectively[154].Onion (Allium cepa) varieties are rich in quercetin (flavonol). Their flavonoids content and antioxidant activity were evaluated using MAE as the extraction technique. The red onion variety exhibited the highest antioxidant activity. According tothe DPPH test, the IC50 was 17.09 mg/cm3 and the total amount of quercetin extracted was 134.7 mg/100 g dried sample[155].In a recent study, the antioxidant activity of polyphenol compounds present in pomegranate (Punica granatum) peels was evaluated using the MAE technique with water as solvent. Pronounced polyphenols yields were reached (210.4 mg GAE/g extract). The IC50 was 14.53 µg/cm3 (DPPH test), confirming an elevated antioxidant capacity of pomegranate extract[156].Figure 2 showsthe free radical scavenging capacity of the pomegranate extract evaluated by the DPPH test.The phenolic compounds extracted by MAE have activity equivalent to the synthetic BHT antioxidant in concentrations over 40 µg/cm3. At this concentration, the antioxidant activity of pomegranate extract and BHT were over 90%, indicating MAE as a potential alternative for obtaining natural antioxidants.The conventional solid-liquid extraction can generate undesirable residues with products. In addition, the extract cansuffer oxidative transformations during the solvent removal step[119]. Several scientific investigations report MAE applicability for obtaining natural antioxidants, without generating undesirable residues, as the extraction of polyphenols from peanut skins[157], whole tomato (Solanum spp.)[101], grape (Vitis vinifera) seeds[158], sweet potato (Ipomoea batatas) leaves[159] and bean (Phaseolus vulgaris L.)[160]. In general, the antioxidant activity of extracts obtained by MAE is higher than the activity of extracts obtained by conventional techniques, because the microwave treatment does not cause any deterioration of antioxidant properties of the extract[161].
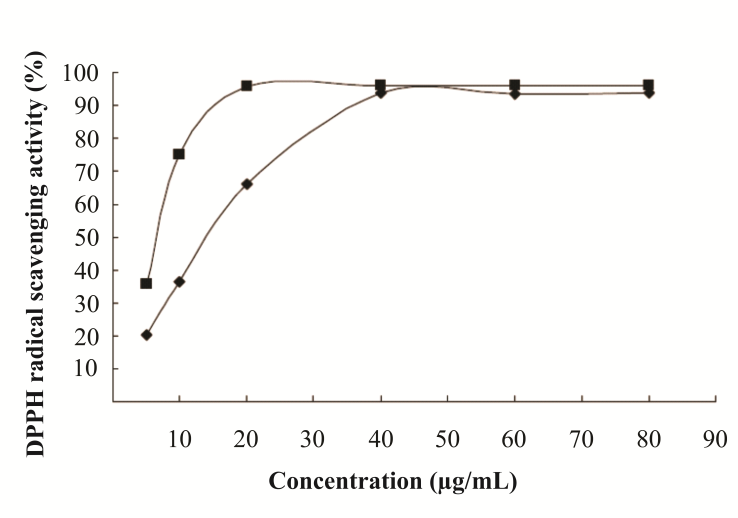 | Figure 2. Free Radical Scavenging Activity of Pomegranate Polyphenols Obtained by Microwave-Assisted Extraction  and of the Synthetic Antioxidant BHT ( and of the Synthetic Antioxidant BHT ( )[156]1 )[156]1 |
3.5. Ultrasound Assisted Extraction (UAE)
- Most of extraction techniques consist of the manipulation of the solvent physical properties to reduce its superficial tension, to increase the solute solubility and to improve the mass transfer rate; in some cases, these manipulations also induce changes in the solvent polarity[109].The UAE technique consists in using mechanic vibrations caused by sound waves with frequencies higher than 20 kHz. Sound waves are intrinsically different from electromagnetic waves, because the latter can propagate through the vacuum, while sound waves need a physical medium to propagate. The mechanic vibrations cause expansion and compression cycles in the medium, creating bubbles which collapse and cause cavitation, instantly creating a high local pressure and intense local heating. These fast changes induce disruption and thinning of the cell membranes, consequently increasing the mass transfer rate of organic substances from the solid matrix to the solvent[109],[163].The advantages of UAE technique include the simplicity of the equipment and the possibility of using different solvents for the extraction, including water-ethanol mixtures[163]. In food and pharmaceutical fields, UAE is used to extract several bioactive compounds from botanic matrices, as flavonoids[104],[164],[165], polyphenols[166], [167], alkaloids[168], terpenoids[169] and anthocyanins [105],[170]. The improvement of extracting bioactive compounds when applying ultrasounds is attributed to the mass transfer rate increase due to the solvent cavitation induced by the ultrasounds wave passing through the medium[154].An efficient extraction procedure for recovering antioxidant compounds from jabuticaba (Myrciaria cauliflora) skins was proposed in literature: 10 min of UAE + conventional agitated bed extraction (ABE). This combination maximized the extraction of polyphenols and resulted in extracts with high antioxidant activity. At 30 min of reaction (based on the couple oxidation of β-carotene and linoleic acid), the antioxidant activity of the extracts obtained using UAE + ABE was over 85%, while the ABE process presented extracts with antioxidant activity under 65%. Furthermore, UAE + ABE was the best option from the economic point of view, because the extract obtained by this combined technique presented the lowest cost of manufacturing (US$ 387.2/kg of crude extract)[61].Considerable concentrations of rosmarinic acid (6.36 mg/cm3) and total phenols (8,790 ppm GAE) were obtained from deoiled rosemary (Rosmarinus officinalis) leaves applying the UAE technique and water as solvent. The high concentration of antioxidants in the extract resulted in high antioxidant activity and the IC50 was 23.6 µg/cm3. The IC50 was measured by DPPH test[154].In extraction of leek (Allium porrum) stem by UAE, 69.5 mg GAE/g extractwas obtained. The antioxidant activity of the extract was compared to the standard antioxidants: vitamin C and BHT. The IC50 value of the ethanolic extract was 61.1 µg/ cm3. Although theIC50 value of the ethanolic extract was higher than the IC50 of vitamin C (IC50 = 10.6 µg/cm3) and BHT (IC50 = 39.2 µg/cm3), the inhibition composition of the ethanolic extract was very low. Thus, the leek extracts can be used in the industry as efficient agents against oxidation[171].A certain amount of water (40% - 60%) should be added to the solvent in order to obtain satisfactory yields in UAE, because water increases the extraction of flavonoids and other polar compounds. Water addition increases the medium relative polarity and facilitates the propagation of the ultrasonic waves[110]. Using UAE with water as solvent was efficient for reaching specific hydroxylation of polyphenols and carotenoids in order to increase their bioactivity[172].Ultrasound is also a broad method that can be done not only with solvent at atmospheric pressure. The combination of UAE followed by re-extraction of obtained extract by SFE was performed aiming to concentrate diterpenes present in sage extract. The diterpenes are generally considered to be responsible for antioxidant activity of the extracted compounds[173].The coupled system of high-intensity ultrasound + SFE is an efficient manner of enhancing mass transfer in extraction processes. In this sense, a supercritical CO2 extraction of oil from particulate almonds was performed using power ultrasonic transducer with a frequency of 20 kHz. The process performance was evaluated, showing that this system conducted to a 30% increased yield[174]. The same procedure was used for obtaining extract from ginger. In the presence of ultrasound within the supercritical medium, both the extraction rate and the yield increased. Generally, the initial stage of extraction, which is controlled by the external mass transfer, is not affected by ultrasound. Nevertheless, in the subsequent stage of extraction, which is controlled by the internal mass transfer, the ultrasound allows an improvement in the yield[175]. Recently, extracts of malagueta pepper containing capsaicinoids were obtained using SFE and SFE assisted by ultrasound. The assays carried out with ultrasound presented a yield 20% higher[176].
3.6. Summary of the characteristics of SFE, PLE, MAE and UAE
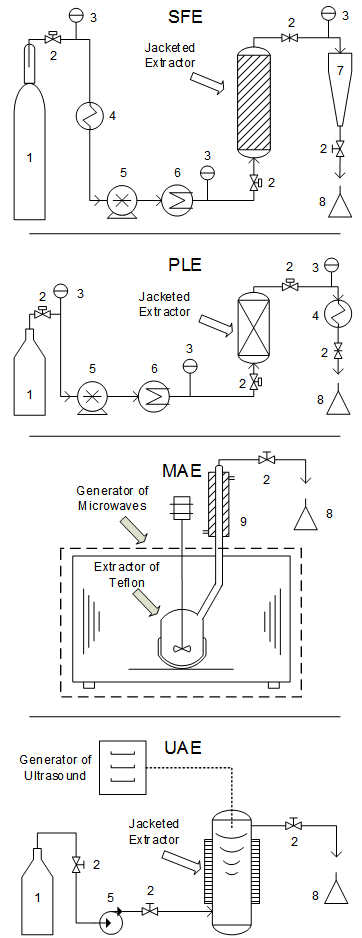
- Table 3 shows compiled information in operational conditions of the four techniques presented for extracting antioxidants. This Table is a compendium of the arguments discussed in the text. The distinguishing characteristics that make each technique more or less attractive for processing different raw materials are also included in Table 3. For instance, SFE technique does not need cosolvent when the target phytochemicals are nonpolar (terpenoids, tocopherols and sitosterols). To extract polyphenols, on the other hand, the use of cosolvents is essential. Thus, the extract quality and yield are closely related to the extraction technique and to the process conditions.Figure 3 exemplifies the novel extraction techniques used for obtaining antioxidants: SFE, PLE, MAE and UAE. The basic schematic diagram of equipment used in each technique is presented. In the case of MAE, the extractor must be built of a material that allows the microwaves to propagate. This material is usually glass or teflon. MAEis mostly performed in batch mode (static), while the other techniques are usually performed in semi-continuous mode (dynamic), using constant solvent flow rate in the extraction vessel. The selection of the way to promote the extractor heating is done by the researchers; in the scheme of Figure 3, the extractors of SFE, PLE and UAE are heated using a jacket.Table 4 shows the antioxidant capacity, expressed as IC50, of compounds obtained from several raw materials using the four novel extraction techniques discussed in the text. Several studies found out high antioxidant capacities of compounds in low concentrations. Certainly, the lower the IC50 value the larger the antioxidant capacity of the extract.
|
|
4. Conclusions
- Obtaining extracts from botanic matrices usingnovelextraction techniques is increasing, andscientific investigations are progressively focusingon the natural antioxidants which are present in these extracts.Antioxidants have been receiving great attention because they bring benefits to the health and food fields. In this overview, the suitability of using each novel technique for obtaining different antioxidant phytochemicals, based on target compound characteristics, was emphasized.
ACKNOWLEDGMENTS
- The authors thank CAPES, CNPq and FAPESP for the financial support. Moyses N. Moraes thanks CAPES and Giovani L. Zabot thanks FAPESP (2011/23665-2) for the Ph.D. assistantships. Juliana M. Prado thanks FAPESP (2010/08684-8) for the postdoctoral fellowship.
Note
- 1. This figure is under the terms of the creative commons attributions license.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML