-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(4): 169-175
doi:10.5923/j.fph.20130304.01
Effect of Walnut (Tetracarpidium conophorum)-oil on Cadmium-Induced Alterations in Lipid Metabolism in Male Albino Rats
Esther O. Abam1, Funmilola Y. Oladipo2, Violette N. Atasie2, Abimbola A. Obayomi1
1Department of Chemical Sciences, Biochemistry option, Bells University of Technology, Ota, Nigeria
2Department of Chemical Sciences, Industrial Chemistry option, Bells University of Technology, Ota, Nigeria
Correspondence to: Esther O. Abam, Department of Chemical Sciences, Biochemistry option, Bells University of Technology, Ota, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
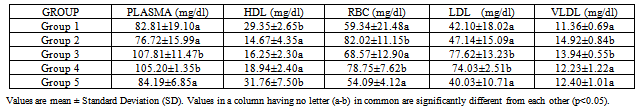
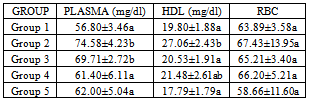
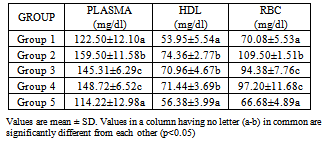
In order to investigate the effects of cadmium exposure on lipid metabolism, and the antidotal efficacy of walnut oil, 35 male rats were divided into 5 groups of 7 animals each. All groups were fed normal rat chow and distilled water or cadmium-poisoned water (200ppm of cadmium as cadmium chloride) for 4 weeks ad libitum. Groups 1 (control) and 2 received distilled water and cadmium-poisoned water respectively. Groups 3 and 4 received cadmium-poisoned water and 2.0g/kg and 4.0g/kg body weight of walnut oil respectively by oral intubation. Group 5 was given distilled water and 2.0g/kg body weight of walnut oil for 4 weeks. The cadmium exposure resulted in disruptions in lipid metabolism in the rats as evidenced in; reduced total and HDL cholesterol, high levels of plasma and HDL triglycerides, high levels of plasma, HDL, RBC phospholipids observed in the cadmium exposed animals compared to control animals. Walnut oil administered at both concentrations restored some of these lipid aberrations while causing an increase in total and LDL cholesterol. This is indicative that the oil could be associated with some cardiovascular risk despite its beneficial role in lowering cadmium levels in blood.
Keywords: Walnut Oil, Cadmium, HDL, Cholesterol, Triglycerides, Phospholipids
Cite this paper: Esther O. Abam, Funmilola Y. Oladipo, Violette N. Atasie, Abimbola A. Obayomi, Effect of Walnut (Tetracarpidium conophorum)-oil on Cadmium-Induced Alterations in Lipid Metabolism in Male Albino Rats, Food and Public Health, Vol. 3 No. 4, 2013, pp. 169-175. doi: 10.5923/j.fph.20130304.01.
Article Outline
1. Introduction
- Heavy metal toxicity has attracted a lot of research interest in recent years[1-9], due to its far-reaching health implications. Consequently, research is on-going as to finding more effective means of managing heavy metal toxicity especially using antioxidant vitamins and natural food substances that will elicit minimum side-effects[1, 10-11]. African walnuts, Tetracarpidium conophorum (Müll. Arg.) Hutch & Dalziel Syn. Plukenetia conophora is one of such plants found to be rich in antioxidants and essential nutrients[12]. The African walnut belongs to the family Euphobiaceae. It is a climber found in the wet part of Southern Nigeria and West Africa. The fruits are greenish with four round seeds in each fruit. The seed testa is hard and the cotyledons are white in colour[13]. Several works have found different parts of this plant to have antioxidant [14], antimicrobial[15], chelating[16] and antidiabetic[17] properties. The fruits are edible; the plant is medicinal and used for various purposes, including masticatory, giddiness, thrush, antihelminthic, toothache, syphilis, dysentery and as an antidote to snake bite[18]. Consequently, we have attempted in this study to use walnut oil in ameliorating some effects of cadmium toxicity.Cadmium (Cd) is the most toxic of the heavy metals with toxicity ten times that of other heavy metals. It is an important environmental pollutant present in soil, water, air and food. Anthropogenic sources add three to ten times more cadmium to the atmosphere than natural sources[19]. Major occupational exposure occurs from non-ferrous smelters during production and processing of Cd, its alloys and compounds and the exposure is increasingly common during recycling of electronic waste. Cd is widely used in industrial processes as an anticorrosive agent, as a stabilizer in PVC products, as a colour pigment, a neutron-absorber in nuclear power plants, and in the fabrication of nickel-cadmium batteries[6]. Phosphate fertilizers also show a big cadmium load. Although some cadmium-containing products can be recycled, a large share of the general cadmium pollution is caused by dumping and incinerating cadmium-containing wastes[20- 21].Cd has no known useful role in higher organisms,[22] although a role for it in lower life forms has been found. A cadmium-dependent carbonic anhydrase has been found in marine diatoms. Cd performs the same function as zinc in these anhydrases[23-24].Cadmium stimulates the formation of metallothioneins (a family of low molecular weight metal binding proteins unique in their high cysteine content) and reactive oxygen species, thus causing oxidative damage to erythrocytes and various tissues resulting in loss of membrane functions[25]. Long term exposure increases lipid peroxidation and causes inhibition of superoxide dismutase activity resulting in oxidative damage to liver, kidney and testes[26].Cadmium toxicity causes hemorrhagic gastroenteritis, liver and kidney necrosis, cardiomyopathy, and metabolic acidosis can also occur. There is some proof that cadmium can also cause cancer[27]. In order to gain insight into the effect of cadmium on lipid metabolism and the possible ameliorative effects, if any, of walnut oil, this study was designed.
2. Methods
2.1. Experimental Animals
- Thirty-five adult male albino rats (Wistar strain) weighing between 120-150g were used for the study. The animals were obtained from the animal house of University of Ibadan, Oyo State, Nigeria. The rats were divided into 5 groups of 7 animals each and kept in separate cages in the animal house of Bells University of Technology, Ota and acclimatized for 2 weeks under normal environmental conditions with 12 hour light/dark cycle. They were fed normal rat chow and water ad libitum. Rat weight was measured with a laboratory electronic scale, which is accurate to within 0.01grams.
2.2. Extraction of Walnut Oil
- The walnuts were purchased from Oja Titun market in Ife, Osun State in Nigeria and were authenticated by Dr. P. I. Oni of the Biological Sciences Department of Bells University of Technology, Ota, Ogun State. Walnuts were separated from their shells, air-dried and milled. Portions of the pulp (about 40g) were extracted at a time with n-hexane using a Soxhlet apparatus. After extraction the solvent was removed yielding the oil. Any remaining solvent in the oil was removed by gentle evaporation over a water bath at 60°C. The oil was stored in the fridge at about 4°C until used. When needed the oil was brought to room temperature before administration.
2.3. Experimental Protocol
- The five groups of rats were treated according to the assay protocol summarized in Table 1. The walnut oil was administered by oral intubation.
2.4. Collection of Experimental Samples
- At the end of four weeks, blood was collected from the animals into heparinized tubes by cardiac puncture under light ether anaesthesia after an overnight fast. Aliquots of blood samples were preserved for cadmium analysis and the remaining was centrifuged immediately at 4000 rpm for 10 minutes to separate plasma and erythrocytes. The plasma was then removed and stored in Eppendorf tubes for further analyses. The erythrocytes were washed with a wash buffer containing 20 mM Tris and 0.15 mM NaCl, pH 7.6. All samples were stored at -20°C until analysed.
2.5. Isolation of High Density Lipoprotein (HDL)
- The HDL fraction was isolated by the method of Gidez et al.,[28] which involved precipitating very low density lipoproteins (VLDL) and low density lipoproteins (LDL) with heparin-manganese chloride (0.06 vol of heparin sodium and 1.0 vol of 1.06M MnCl2) solution. Heparin - manganese chloride solution 0.025 ml was added to 0.25 ml of plasma in a test tube. The resultant mixture was vortexed and left to stand at room temperature for 10 minutes. The mixture was then centrifuged at 4000rpm for 10 minutes. The supernatant (HDL fraction) was carefully decanted into clean Eppendorf tubes and stored at -20 ºC until analysed.
2.6. Extraction of Phospholipids from Plasma, Erythrocytes and HDL
- Plasma lipids were extracted using chloroform-methanol mixture (2:1, v/v) as described by Folch et al.[29]. Briefly, to 0.1ml of plasma in an Eppendorf tube was added 0.9ml of the chloroform-methanol mixture (2:1 v/v). The mixture was then vortexed thoroughly and allowed to stand at room temperature for 30 minutes. It was centrifuged for 10 minutes and the chloroform layer was transferred into a separate tube using a syringe. This represented the lipid extract. Extraction of lipids from erythrocytes and HDL fraction also followed the same procedure as described for plasma but for erythrocytes, chloroform-isopropanol mixture (7:11, v/v) was used according to the method of Rose and Oklander[30].
2.7. Biochemical Analyses
2.7.1. Determination of Cholesterol in Plasma, HDL and Erythrocytes
- Plasma and HDL cholesterol were determinedspectrophotometrically according to the methods of Allain et al.[31] as outlined in the Cromatest diagnostic kits. The reagent was made up of three enzymes: cholesterol esterase (CE), cholesterol oxidase (CO) and peroxidase (POD), and two substrates 4-aminoantipyrine (4 -AA) and phenol. Cholesterol and its esters are released from thelipoproteins through the action of a detergent. CE then hydrolyses the cholesteryl esters to cholesterol and fatty acids. Subsequent enzymatic oxidation of cholesterol by CO results in the production of H2O2. The H2O2 produced condenses with phenol in a reaction catalysed by a peroxidase. Aquinoneimine dye is formed whose concentration is proportional to the concentration of cholesterol in the sample and read at 550nm against reagent blank. For cholesterol in erythrocytes, 0.1ml of the erythrocyte lipid (chloroform/methanol) extract was pipetted into different test tubes and evaporated to dryness at 60ºC. The dried extract was re-dissolved in 20 µl of a triton-X/chloroform mixture (1:1, v/v) and evaporated again as before. 1ml of the cholesterol reagent was then added to the dried extract, vortexed and taken through the procedure outlined in the kit.
2.7.2. Determination of Triglycerides in Plasma, HDL and Erythrocytes
- Plasma and HDL triglycerides were determined spectrophotometrically by the method of Buccolo and David[32], as outlined in the Cromatest diagnostic kit. The method was based on the enzymatic hydrolysis of plasma triglycerides to glycerol and free fatty acids (FFA) by lipoprotein lipase (LPL). The glycerol was phosphorylated by adenosine triphosphate (ATP) in the presence of glycerolkinase (GK) to form glycerol-3-phosphate (G-3-P) and adenosine diphosphate (ADP). G-3-P was oxidized by glycerophosphate oxidase (GPO) to form dihydroxy acetone phosphate (DHAP) and hydrogen peroxide.A red chromogen produced by the peroxidase (POD)-catalysed coupling of 4- aminoantipyrine (4-AA) and phenol with hydrogen peroxide (H2O2) was proportional to the concentration of triglyceride in the sample.For erythrocyte triglycerides, 0.1ml of the erythrocyte lipid extract was used. 0.1 ml of the chloroform-isopropanol extract was pipetted into test tube and evaporated to dryness at 60ºC. The dried extract was re-dissolved in 200 µl of 95% ethanol. Then 1ml of the triglyceride reagent was added and vortexed. After incubation in the dark at room temperature for 30 minutes, absorbance was read at 500 nm against reagent blank.
2.7.3. Determination of Plasma Phospholipids
- Plasma phospholipids were determined according to the method of Stewart[33]. The method is based on complex formation between ammonium ferrothiocyanate and phospholipids. 100 µl of plasma was extracted with 0.9ml of chloroform - methanol mixture (2:1,v/v). 0.1 of this chloroform lipid extract (3.7.3) was evaporated to dryness at 60ºC. The dried extract was then dissolved in 2ml chloroform. 2ml ammonium ferrothiocyanate was added and mixed well. The chloroform layer was removed using a syringe and the absorbance read at 488 nm against a blank. The blank was prepared by mixing 2ml chloroform with 2 ml ammonium ferrothiocyanate in a dry tube, and the chloroform layer removed and used as the blank.
2.7.4. Determination of Erythrocyte Phospholipids
- 0.1 ml of the erythrocyte lipid extract was taken in a test tube and evaporated to dryness at 60ºC. 2 ml of chloroform and 2ml of ammonium ferrothiocyanate were added to the extract and vortexed. The chloroform layer was removed using a syringe and the absorbance was read at 488 nm.
2.7.5. Determination of HDL Phospholipids
- HDL phospholipids were determined in the HDL fraction using the same procedure for plasma.
2.7.6. Calculation of LDL and VLDL Cholesterol
- LDL and VLDL cholesterol values were calculated using the Friedewald equation[34] which calculates these values using analysed values of Total cholesterol, HDL cholesterol and Triglycerides.LDL-Cholesterol = Total Cholesterol – HDL- Cholesterol – Triglycerides/5
2.7.7. Determination of Cadmium in Blood
- Blood cadmium was determined by atomic absorption spectrometry (Thermo Scientific Equipment S-series (model S4 AA system)). Concentrated nitric acid was used for the digestion of the blood samples which was then read with the AAS.
2.7.8. Statistical Protocol
- Results are expressed as mean ± S.D. One way analysis (ANOVA) followed by Duncan’s test was used to analyse the results with p<0.05 considered significant.
3. Results
|
|
|
|
4. Discussion
- The use of traditional medicine and medicinal plants in Africa and Nigeria specifically as a normal approach to the maintenance of health is an age-long approach and is gaining more awareness due to its efficacy and recent advances in research in this area[14-15, 17, 35]. This present study was carried out to investigate the effect of walnut oil, a well-known fruit with antioxidant properties, on cadmium-induced alterations in lipid metabolism.In comparison to controls, rats poisoned with cadmium in this study displayed lower HDL-cholesterol concentrations in plasma. This was also associated with increase in triglycerides or hypertriglyceridemia and increase in phospholipids. Evidence for further cadmium-induced disruptions in lipid metabolism is shown in the increase in cholesterol and phospholipids in the red blood cells without a concomitant increase in triglycerides; high levels of plasma and HDL triglycerides; high levels of plasma, HDL, RBCphospholipids observed in the cadmium-exposed animals compared to control animals. HDL enables lipids like cholesterol and triglycerides to be transported within the water-based bloodstream. In healthy individuals, about thirty percent of blood cholesterol is carried by HDL. Blood tests typically report HDL-C level, i.e. the amount of cholesterol contained in HDL particles. It is often contrasted with low density or LDL cholesterol or LDL-C. HDL particles are able to remove cholesterol from within artery atheroma and transport it back to the liver for excretion or re-utilization, which is the main reason why the cholesterol carried within HDL particles (HDL-C) is sometimes called "good cholesterol" (despite the fact that it is exactly the same as the cholesterol in LDL particles). Those with higher levels of HDL-C seem to have fewer problems with cardiovascular diseases, while those with low HDL-C cholesterol levels (less than 40 mg/dL or about 1 mmol/L) have increased rates for heart disease,[36- 37]. Because LDL particles appear harmless until they are within the blood vessel walls and oxidized by free radicals,[38], it is postulated that ingesting antioxidants and minimizing free radical exposure may reduce LDL's contribution to atherosclerosis, though results are not conclusive[39]. Walnuts have been found to be rich in antioxidants[14]. In this study, the walnut oil proved to be quite effective in lowering the increased RBC cholesterol and the increased triglycerides in the plasma and HDL fraction. This was also the trend concerning the observed phospholipidosis in the plasma and RBC. Administration of the lower concentration of the oil reversed this increased levels of phospholipids. Increased cadmium in the blood was significantly reduced upon administration of the oil which might be a pointer to the fact that the oil could possess some chelating properties. This is in consonance with the work of Olabinri et al.[16], who observed a dose-dependent increase in the chelating properties of the aqueous fraction of walnut in vitro.On the other hand, administration of the walnut oil appeared to lead to increase in LDL levels in the cadmium exposed animals in this study which implies an exacerbating effect on cadmium toxicity. The oil seemed to raise LDL cholesterol above the levels found in the cadmium-exposed animals upon its administration. This might be a pointer to the fact that the oil might not be so effective in the amelioration of cadmium toxicity despite its other useful effects. This is contrary to the host of studies that have shown that increasing the dietary intake of monounsaturated-dense walnuts has favourable effects on cholesterol levels and other cardiovascular risk factors. In one such study, involving an 8-week crossover feeding trial in subjects with moderate hypercholesterolemia, it was found thatsubstituting walnuts for 32% of the energy from monounsaturated fatty acids in a cholesterol-lowering Mediterranean diet improves vascular endothelial function[40]. A lot of these studies however dealt with the whole nut in a diet-based study and not the oil alone like was used in this study and there was no heavy metal introduced[41-45].
5. Conclusions
- Further research into the use of walnut oil in diets is needed in order to authenticate these results although we postulate that incorporating the whole fruit in a diet-based study might be more effective in appropriating the antioxidant properties of the fruit more than extracting the oil and ingestion. This could account for the disparity in results of this study and other studies that have shown the fruit to lower LDL levels.
ACKNOWLEDGEMENTS
- The authors are grateful to the staff of the Chemical Sciences Department of Bells University of Technology for their support during this research, especially Mrs. T.O. Ogunbiyi, Mr. Steve Oyelowo and Mr. Clement Ogunbona for their technical assistance and also, Dr. P. I. Oni of the Biological Sciences Department, also of Bells University of Technology.
References
| [1] | Abam E., Okediran B.S., Odukoya O.O., Adamson I., Ademuyiwa O., 2008, Reversal of Ionoregulatory Disruptions in Occupational Lead Exposure by Vitamin C, Environ. Toxicol. Pharmacol |
| [2] | Ademuyiwa O., Ugbaja R.N., Idumebor F., Adebawo O., 2005a, Plasma lipid profiles and risk of cardiovascular disease in occupational lead exposure in Abeokuta, Nigeria, Lipids Health Dis, 4, 19 |
| [3] | Ademuyiwa O., Ugbaja R.N., Ojo D.A., Owoigbe A.O., Adeokun S.E., 2005b, Reversal of aminolevulinic acid dehydratase (ALAD) inhibition and reduction of erythrocyte protoporphyrin levels by vitamin C in occupational lead exposure in Abeokuta, Nigeria. Environ. Toxicol. Pharmacol. 20, 404-411 |
| [4] | Ademuyiwa O., Agarwal R., Chandra R., Behari J.R., 2009, Lead-induced phospholipidosis and cholesterogenesis in rat tissues, Chemico-Biological Interactions. 179(2-3), 314-320 |
| [5] | Okediran B.S., Abam E., Odukoya O.O., Adamson I., Ademuyiwa O., 2009, Membrane, intracellular, plasma and urinary sodium and potassium in occupational lead exposure. effects of vitamin C supplementation, Trace Elements and Electrolytes, 26, 49-59 |
| [6] | Godt J., Scheidig F., Grosse-Siestrup C., Esche V.,Brandenburg P., Reich A., Groneberg DA., 2006, The toxicity of cadmium and resulting hazards for human health, J of Occup Med and Toxicol, 1, 22 |
| [7] | Lindh U., Hudecek R., Danersund A., Eriksson S., Lindvall A., 2002, Removal of dental amalgam and other metal alloys supported by antioxidant therapy alleviates symptoms and improves quality of life in patients with amalgam-associated ill health, Neuroendocrinol Lett, 23, 459–82 |
| [8] | Zhang F.Q., Wang Y.S., Lou Z.P., Dong J.D., 2007, Effect of heavy metal stress on antioxidative enzymes and lipid peroxodation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza), Chemosphere, 67 (1), 44-50 |
| [9] | Metwally M.A.A. and Fouad I.M., 2008, Biochemical changes induced by heavy metal pollution in marine fishes at Khomse Coast, Libya, Global Verterinaria, 2 (6), 308- 311 |
| [10] | Al-Hashem F., Dallak M., Bashir N., Abbas M., Elessa R., Khalil M. and Al-Khateeb M., 2009, American Journal of Pharmacology and Toxicology 4 (3), 107-117. |
| [11] | Gaurav D., Preet S. and Dua K.K., 2010, Chronic Cadmium toxicity in Rats; Treatment with combined administration of vitamins, amino acids, antioxidants and essential metals, Journal of Food and Drug Analysis, 18(16), 464-470. |
| [12] | Ayoola P.B., Adeyeye A., Onawumi O.O. and Faboya O.O.P., 2011, Phytochemical and nutrient evaluation ofTetracarpidium comophorum (Nigerian walnut) root, IJRRS, 7 (2),197-202 |
| [13] | Ehiagbanare J.E., Onyibe H.I., 2007, Effect of pre-sowing treatments on seed germination and seedling growth of Tetracarpidium conophorum Mull, African Journal of Biotechnology 6 (6), 697–698 |
| [14] | Amaeze O.U., Ayoola G.A., Sofidiya M.O., Adepoju-Bello A.A., Adegoke A.O. and Coker H.A.B., 2011, Evaluation of Antioxidant activity of Tetracarpidium conophorum (Müll. Arg). Hutch & Dalziel Leaves. Oxidative Medicine and Cellular Longevity, xx-xx. |
| [15] | Ajaiyeoba E.O., Fadare D.A., 2006, Antimicrobial potential of extracts and fractions of the African walnut — Tetracarpidium conophorum, Afr. J. Biotechnol. 5(22), 2322–2325 |
| [16] | Olabinri B.M., Eniyansoro O.O., Okoronkwo C.O., Olabinri P.F. and Olaleye M.T., 2010, Evaluation of aqueous extract of Tetracarpidium conophorum (African walnut) in vitro, Int J of App Res in Nat Products, 3 (3), 13-18 |
| [17] | Odoemena C.S.I., Udosen I.R. and Sam S.M., 2010, Antidiabetic activity of Tetracarpidium conophorum Muell Arg. (Hutch & Dalz) ethanolic seed extract on diabetic rats, Advances in Science and Technology, 4(2), 120-124 |
| [18] | Odugbemi O. and Akinsulire O., 2008, Medicinal plants by species names In: Outlines and Pictures of Medicinal Plants from Nigeria, Odugbemi, T. (Ed). University of Lagos Press, Lagos, Nigeria. 112 |
| [19] | Irwin R.J., Van Mouwerik M., Stevend L., Seese M.D. and Basham W., 2003, Environmental contaminants encyclopedia, National Park Service, Water Resources Division, Fort Collins, Colorado. Distributed within the federal government as electronic document |
| [20] | Jarup L., Hazards of heavy metal contamination, 2003, Br. Med. Bull, 68, 168-182 |
| [21] | Borde A.U., Athawaley A.M., Mendhe M.S., Patil M.K., Lokhande P.R. and Jaiswal S.A., 2008, Ameliorating potential of Ashwangandha on cadmium chloride induced changes in weights of visceral organs, Verterinary World, 1 (11), 343-345 |
| [22] | Michael C.H., Heavy metal Encyclopedia of Earth. National Council for Science and the Environment. Monosson E. and Cleveland C. (Eds) Washington DC. 2010 |
| [23] | Lane T.W. and Morel F.M., 2000, A Biological Function for Cadmium in Marine Diatoms. Proc. Natl. Acad. Sci, 97 (9), 4627–4631 |
| [24] | Lane T.W., Saito M.A., George G.N., Pickering I.J., Prince R.C. and Morel F.M.M., 2005, A Cadmium Enzyme from a Marine Diatom Nature, 435 (42), 42 |
| [25] | Sakar S., Yadav P. and Bhatnagar D., 1998, Lipid peroxidative damage on cadium exposure and alterations in antioxidant system in rat erythrocytes: a study with relation to time, Biometals, 11(2), 153-157 |
| [26] | Patra R.C., Swarup D. and Senapat S.K., 1999, Effects of cadmium on lopid peroxides and superoxide dismutase in hepatic, renal and testicular tissue of rats, Veterinary and Human Tox, 41(2), 65-67 |
| [27] | Waalkes M.P., Rehm S., Riggs C.W., Bare R.M. and Devor D.E. et al., 1998, Cadmium carcinogenesis in male Wistar[Crl:(WI)BR] rats: dose-response analysis of tumor induction in the prostate and testes and at the injection site, Cancer Res, 48(16), 4656-4663 |
| [28] | Gidez L.I., Miller G.J., Burstein M., Slagle S. and Eder H.A., 1982, Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure, J. Lipid Res, 23, 1206-1223 |
| [29] | Folch J., Lees M. and Sloane Stanley G.H., 1957, A simple method for the isolation and purification of total lipids from animal tissues, J. Biol. Chem., 226, 497-509 |
| [30] | Rose H.G. and Oklander M., 1965, Improved procedure for the extraction of lipids from human erythrocytes, J. Lipid Res, 6, 428-431 |
| [31] | Allain C.C., Poon L.S., Clau C.S.G., Richmond W.Y. and Fu P.D., 1974, Enzymatic determination of total serum cholesterol, Clin. Chem., 20, 470-478 |
| [32] | Buccolo G. and David H., 1973, Quantitative determination of serum triglycerides by the use of enzymes, Clin. Chem. 19, 476-478 |
| [33] | Stewart J.C.M., 1979, Colourimetric determination of phospholipids with ammonium ferrothiocyanate, Anal. Biochem 104, 10-14 |
| [34] | Friedewald W.T., Levy R.I., Fredrickson D.S., 1972, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge, Clin. Chem., 18, 499-502 |
| [35] | Farombi E.O., 2011, Recent Advances in Nutrition Research: Nutraceuticals, Nutrigenomics and Chemoprevention.Transaction of the Nigerian Society of Biochemistry and Molecular Biology, 1 (1),s 44-65 |
| [36] | Toth P., 2005, The "Good Cholesterol" High-Density Lipoprotein", Circulation, 111, e89-e91 |
| [37] | Walter M.F., 2007, The role of hypertriglyceridemia in atherosclerosis, Curr. Atheroscler Rep, 9, 110-5 |
| [38] | Teissedre P.L., Frankel E.N., Waterhouse A.L., Peleg H. and German J.B., 1996, Inhibition of in vitro human LDL oxidation by phenolic antioxidants from grapes and wines, J Sci Food Agric, 70 (1), 55-61 |
| [39] | Esterbauer H., Puhl H., Dieber-Rotheneder M., Waeg G., Rabl H., 1991, Effect of antioxidants on oxidative modification of LDL, Annals of Medicine, 23 (5), 573–81 |
| [40] | Ros E., Nunez I., Perez-Heras A., Serra M., Gilabert R., Casals E. and Deulofeu M.D., 2004, A Walnut Diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial, American Heart Association Inc., 1-4 |
| [41] | Zambón D., Sabaté J. and Muñoz S. et al., 2000, Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women: a randomized crossover trial, Ann Intern Med., 132, 538–546 |
| [42] | Sabaté J., Fraser G.E. and Burke K. et al. 1993, Effects of walnuts on serum lipids levels and blood pressure in normal men, N Engl J Med., 328, 603–607 |
| [43] | Kris-Etherton P.M., Yu-Poth S., Sabaté J., et al. 1999, Nuts and their bioactive constituents: effects on serum lipids and other factors that affect disease risk. Am J Clin Nutr, 70(3), 504S–511S |
| [44] | Kris-Etherton P.M., Zhao G. and Binkoski A.E., et al. 2001, The effect of nuts on coronary heart disease risk, Nutr Rev 59, 103–111 |
| [45] | Iwamoto M., Imaizumi K. and Sato M. et al., 2002, Serum lipid profiles in Japanese women and men during consumption of walnuts, Eur J Clin Nutr, 56, 629–637 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML