-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(3): 130-136
doi:10.5923/j.fph.20130303.03
Antioxidative Effect of Tocotrienol Rich Fraction from Palm Fatty Acid Distillate on Oxidative Stress
Kgs Ahmadi1, Sri Kumalaningsih2, Susinggih Wijana2, Imam Santoso2
1Agroindustry Technology, Agriculture Postgraduate Program, Brawijaya University, Malang, 65141, Indonesia
2Department of Agroindustry Technology, Brawijaya University, Malang, 65141, Indonesia
Correspondence to: Kgs Ahmadi, Agroindustry Technology, Agriculture Postgraduate Program, Brawijaya University, Malang, 65141, Indonesia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This research aimed to study the antioxidative effect of tocotrienol rich fraction (TRF) from palm fatty acid distillate (PFAD) on oxidative stress of Wistar strain male rats.The rats were treated by highly oxidized frying oil to induce oxidative stress. The rats were grouped into 6, i.e. control group (non oxidative stress), group treated by highly oxidized frying oil (oxidative stress group), and 4 groups treated by highly oxidized frying oil + TRF 25 mg/kg bw, 50 mg/kg bw, 75 mg/kg bw, and commercial vitamin E 75 mg/kg bw, respectively.The results showed that TRF and commercial vitamin E significantly (p<0.05) reduced oxidative stress level.Rats fed by TRF 50 mg/kg bw showed the best oxidative stress improvement, indicated by the highest increase in blood serum SOD activity (6.2 unit/ml), as well as the highest decrease in blood serum MDA level (47.3 ng/ml).
Keywords: Oxidative Stress, Tocotrienol, Palm Fatty Acid Distillate, Vitamin E
Cite this paper: Kgs Ahmadi, Sri Kumalaningsih, Susinggih Wijana, Imam Santoso, Antioxidative Effect of Tocotrienol Rich Fraction from Palm Fatty Acid Distillate on Oxidative Stress, Food and Public Health, Vol. 3 No. 3, 2013, pp. 130-136. doi: 10.5923/j.fph.20130303.03.
Article Outline
1. Introduction
- Palm fatty acid distillate (PFAD) is a by-product of deodorization in physical refining of palm oil.The major component of PFAD is free fatty acid, oxidation products as minor component, and it also contains some health beneficial trace components such as tocopherols, tocotrienols, phytosterols, and squalene.Liu et al.[1] reported that PFAD has tocopherol and tocotrienol content in the range of 0.7-1%, and it is considered as one of important sources of natural vitamin E.Tocopherols and tocotrienols have 4 isomers, α, β, γ, δtocopherol and α, β, γ, δtocotrienol, and all of them possess antioxidant activity[2].Ng et al.[3] revealed that palm oil contains α tocopherol, α tocotrienol, γ tocotrienol, and δ tocotrienol. Our previousstudy[4] showed that PFAD has α tocopherol (33.84%), α tocotrienol (21.27%), γ tocotrienol (38.49%), and δ tocotrienol (6.40%).Tocopherols and tocotrienols act as antioxidant by scavenging reactive free radicals and convert them into more stable products.Each vitamin E isomershas different antioxidant activity.According to Watson andPreed[5], compared to αtocopherol, αtocotrienol has 50 times greater antioxidant activity on Fe induced lipid peroxidation and 6.5 times greater in protecting cytochrome P-450 from oxidativedamage.Lipidperoxidation inhibition by lipophillic antioxidant such as vitamin E leadsto oxidative stress depletion. Tocotrienols are more effective as antioxidant than tocopherol due to fast absorption into bloodstream and rapidly circulated into liver[6].Invitro study revealed that tocotrienol has very good antioxidant activity by suppressing reactive oxygen species production[7].Oxidative damage is initiated by oxidative stress. In biological system, oxidative stress occurs due to imbalance activity of oxidizing species and cell antioxidant defense system, and it is triggered by excessive free radicals.The source of free radicals is reactive oxygen species (ROS).Basically, human body has antioxidant defense mechanism that comprises ofantioxidant enzymes such as superoxide dismutase (SOD), gluthation peroxidase (GPx), and catalase (CAT), and antioxidant vitamins such as vitamin E and vitamin C[8,9,10].These enzymes and antioxidants have a role to reduce free radicals that naturally produced.SOD is effectively scavenges ROS and inhibits lipid peroxidation.Anion superoxide radical (O2•-) is dismutased by SOD to produce hydrogen peroxide (H2O2) that further decomposed by catalase.Due to its role in converting O2•- to H2O2, SOD is an important enzyme of antioxidant defensesystem[11].In normal condition, there is a balance between ROS formation and cell antioxidant activity. However, the impairment of this balance leads to oxidative stress that further implies to cell component damages[12].One of oxidative stress damages is lipid peroxidation that produces lipid peroxides.These compounds further degrade into some molecules such as epoxides, hydrocarbons, and aldehydes, and malondialdehyde (MDA) is one indicator of lipid peroxidation due to oxidative stress[13,14].In oxidative stress condition, our body requires additional antioxidant. Due to its antioxidant activity, vitamin E has the ability to prevent oxidative stress. Eder et al.[15] showed that αtocopherol 25 to 250 mg/kg reduced negative effect of thermal oxidized oil as one of oxidative stress causes. Furthermore, Fairus et al.[16] explained that tocotrienols are more effective than tocopherols in reducing and inhibiting lipid peroxidation due to their intramembrane mobility and higher reactivity with free radicals. Therefore, this researchaimed to study the effect of various doses of TRF on reducing oxidative stress.It was also objected to compare the capability of TRF and commercial vitamin E (αtocopherol) in diminishing oxidative stress.
2. Materials and Methods
2.1. Materials
- PFAD was kindly obtained from a palm oil manufacturer, PT SalimIvomasPratama, Surabaya, East Java, Indonesia, highly thermal oxidized frying oils with peroxide value of 102.3 meq/kg, vitamin E standards (αtocopherol from Nacalai, Japan; αtocotrienol, γtocotrienol, and δtocotrienol from Santa Cruz Biotechnology Inc.), technical grade KOH and hexane, DPPH, and other pro analysis chemical reagents from Merck.
2.2. TRF Preparation
- This method was referred to Ahmadiet al.[4]. Briefly, TRF preparation was as followed: PFAD was saponified by using KOH and the soap was separated from unsaponifiable fraction by solvent extraction.Unsaponifiable fraction was further crystallized by low temperature solvent crystallization. Crystal fraction and the solvent were separated and vacuum evaporation was performed to obtain TRF.
2.3. Experimental Design
- This research used 24 rats (2 month-old, 200 g body weight) that divided into6 groups and each group comprised of 4 rats. These groups were: control group (non oxidative stress group), group treated by highly thermal oxidized frying oil (oxidative stress group), and 4 groups treated by highly thermal oxidized frying oil + TRF 25 mg/kg bw, 50 mg/kg bw, 75 mg/kg bw, and commercial vitamin E 75 mg/kg bw.The teratment of TRF wirh doses of 25-75 mg/kg bw was based on the RDA (Recommended Daily Allowance) for European of 10 mg [17] and 10-50 mg/kg bw/day[18]. Average body weight of rats was 200 g, therefore to obtain TRF dose of 25 mg/kg bw, we diluted 5 mg TRF in 1 ml of palm oil. Similarly, we diluted 10 mg TRF in 1 ml of palm oil and 15 mg in 1 ml of palm oil for TRF dose of 50 mg/kg bw and 75 mg/kg bw, respectively.Each group was fed by modified commercial feeding that had protein 5.56%, fat 1.13%, and carbohydrate\ 18.05%. The rats were adapted to feed and laboratory environmentfor seven days.Oxidative stress induction was conducted by force feeding of highly thermal oxidized frying oil that had peroxide value 102.3 meq/kg with dose of 2 ml/day/rat for seven days.The experiment was conducted for 28 days andblood sample was taken from every rat by retro orbitalplexus at week 0 (before treatment) and week 4 (after treatment) for blood serum SOD and MDA analysis.Each group of rats was force fed by various doses of TRF or commercial vitamin E, accordingly. This experiment was approved for ethical clearance from Animal Care and Use Committee, Brawijaya University with ethical clearance No. 83-KEP-UB.
2.3.1. Blood Serum SOD Activity Analysis
- Blood serum SOD activity analysis was referred to method of Spitz and Oberley[19] with some modifications. This analysis was colorimetric SOD assayed by using NBT. Basic principle of this method was xanthine-xanthine oxidase was utilized to generate a superoxide flux.NBT reduction by O2- to blue formazan was followed at 580 nm in a Shimadzu spectrophotometer at room temperature. When blood serum (containing SOD activity) was added to the system, the rate of NBT formation was progressively inhibited.The amount of inhibition was defined as percentage of the reference rate when SOD activity was not present. The data were plotted as percentage inhibition vs blood serum volume. One unit of activity was defined as the amount of serum volume needed to decrease the reference rate of 50% of maximum inhibition.The analysis was conducted as followed: blood was centrifuged at 3000 rpm for 10 min and subsequently serum was centrifuged again at 6000 rpm for 10 min.Supernatant 100 μl was mixed with 100 µL xanthine, 100 µL xanthine oxidase, 100 µL NBT, and 600 µL PBS. This mixture was incubated at 30℃ for 30 min until the colour change to purple. The absorbance was measured at λ=580 nm.
2.3.2. Blood Serum MDA Level Analysis
- This method was referred to method of Pasha and Sadavisadu[20]. MDA analysis is measuredmalondialdehyde as secondary product of lipid peroxidation that reacts with TBA in acidic condition (pH 2-3) at 97-100C to form pink colour that measured at 532 nm. Blood was centrifuged at 4000 rpm for 10 min.Blood serum of250 μL was added by 625 μL TCA 10% and HCl 1 N 100 μL, and the mixture was shaked until homogen.Aquabidest of 0.5 mL was added and the mixture was shaked, and subsequently added by Na-thiol 1% 100 μL and homogenized.Then, the mixture was heated in water bath shaker at 100C for 30 min before centrifugation at 3000 rpm for 15 min. Supernatant was added by aquabidest 3 mL and the absorbance was measured at 532 nm.
2.4. Vitamin E analysis
- Analysis of vitamin E was referred to method of Ball[21] by using HPLC (Shimadzu LC20AT).The column used was VP-ODS 4.6 mm 25 mm, detector UV (SPD 20A).Each sample of PFAD, unsaponifiable fraction of PFAD, and TRF were diluted in methanol at concentration of 1000 ppm.After sieving by milipore 0.4 μm, each sample was injected into HPLC column at injection volume of 100 μL.HPLC operational conditions were mobile phase of methanol: water 95:5 at flowrate of 1 ml/min.Each standard of vitamin E was separately injected into HPLC column for identification and quantification of each identified vitamin E isomers.
2.5. Antioxidant Activity Analysis
- Antioxidant activity was assayed by method of Kim[22]. Sample of 5 mg was diluted in 10 ml chloroform:methanol (2:1 v/v).As much as 1 ml DPPH solution 0.2 mM in methanol was added into 4 ml sample solution. The mixture was let for 30 min at room temperature before absorbance measurement at λ=517 nm. Radical scavenging activity was expressed as the percentage of DPPH color reduction by using following equation:Radical scavenging activity (%) =
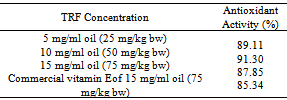
 A0 = blank absorbanceA1 = sample absorbanceAntioxidant activity of TRF at various doses that fed to rats was measured at TRF concentration of 5 mg/ml oil (TRF dose of 25 mg/kg bw), 10 mg/ml oil (TRF dose of 50 mg/kg bw), and 15 mg/ml oil (TRF and commercial vitamin E dose of 75 mg/kg bw, separately).Each concentration of TRF or commercial vitamin E in oil was taken for 5 ml and then diluted with 10 ml chloroform: methanol (2:1 v/v) and experienced similar preparation as previously explained.
A0 = blank absorbanceA1 = sample absorbanceAntioxidant activity of TRF at various doses that fed to rats was measured at TRF concentration of 5 mg/ml oil (TRF dose of 25 mg/kg bw), 10 mg/ml oil (TRF dose of 50 mg/kg bw), and 15 mg/ml oil (TRF and commercial vitamin E dose of 75 mg/kg bw, separately).Each concentration of TRF or commercial vitamin E in oil was taken for 5 ml and then diluted with 10 ml chloroform: methanol (2:1 v/v) and experienced similar preparation as previously explained.2.6. Statistical Analysis
- Data obtained was analysis by using analysis of variance and Duncan test p<0.05 to know the differences among treatments.
3. Results and Discussions
3.1. PFAD and TRF Characteristics and in vitro Antioxidant Activity
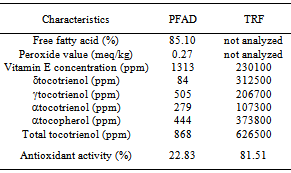
- PFAD had high vitamin E concentration of 1313 ppm, that was higher than other sources of vitamin E such as rice bran (300 ppm)[23], cereals and berry of84-318 and 56-140 ppm[24], and olive oil (100-270 ppm)[25].Vitamin E composition of PFAD was αtocopherol 33.48%, αtocotrienol 17.57%, δtocotrienol 29.06%, and γtocotrienol 19.89% (Table 1). Tocotrienol was the major vitamin E compounds in PFAD with its quantity of 66.52%, and theremain was tocopherol as αtocopherol of 33.48%. The results was in accordance to that reported by Musalmah et al.[26] that PFAD contains tocotrienol (70%) and tocopherol (30%). Meanwhile, Puahet al.[27] reported that vitamin E composition of refined palm oil is α tocopherol (14–17%), α tocotrienol (22–24%), γ tocotrienol (49–53%), δ tocotrienol (6–7%) and αtocomonoenol (3%). Total tocotrienol of PFAD in this study was lower than refined palm oil that was supposed due to thermal degradation during deodorization. Based on its molecular structure, tocotrienol has three double bonds in its phytil side chain that influences its thermal stability.Tocotrienol rich fraction from low temperature solvent crystallization had vitamin E composition as followed: αtocopherol 37.38% (373800 ppm), αtocotrienol 10.73% (107300 ppm), γtocotrienol 20.67% (206700 ppm), and δtocotrienol 31.25% (312500 ppm) with vitamin E concentration of 23.01 g/100 g. TRF revealed high in vitro antioxidant activity of 81.51% and it was an evidence that TRF was capable to stabilize free radical DPPH. The ability of vitamin E to scavenge free radical is related to their ability to stabilize free radicals by donating hydrogen atom. According to Seppanenet al.[28], vitamin E (tocopherols and tocotrienols) is an antioxidant that capable to donate phenolic hydrogen to lipid free radicals and retards autocatalytic reaction of lipid peroxidation. Shahidi and Zhong[29] showed that tocopherols and tocotrienols of vegetable oils are lipid soluble antioxidant that effectively scavenges lipid peroxyl radicals.Similarly, Zempleniet al.[30] explained that vitamin E has strong ability to scavenge peroxyl radicals (ROO•) in protecting polyunsaturated fatty acids (PUFA) and peroxyl radicals are 1000 times faster to react with vitamin E rather than PUFA.
|
|
3.2. Serum SOD Activity
- Superoxide dismutase (SOD) is an intracellular enzymatic primary antioxidant that protect cell by scavenging free radicals.SOD activity is a marker of antioxidant status of the body anda bioindicator of oxidative stress. In this study, oxidative stress was induced by feeding highly thermal oxidized frying oil that was heated several times at 190⁰C. This oil had primary and secondary products of oxidation and acted as free radical sources that caused lipid peroxidation. The effectiveness of TRF in reducing lipid peroxidation due to ingestion of free radicals from highly thermal oxidized oil was elucidated in this study. Eder et al.[15] reported that PUFA membrane peroxidation and lipid peroxidation product formation occurred due to thermal oxidized frying oil ingestion.Highly thermal oxidized frying oil used in this study was brown in colour, had rancid odour, and had peroxide value of102.03 meq/kg. Eder et al.[15] reported that thermal oxidized frying oil had 200 times greater of peroxide value and 15 times greater of polar compounds than fresh oil due to hydrolysis, oxidation, and polymerization reaction.This oil also contained very labile primary oxidation products (peroxides) that could be further oxidized into secondary oxidation products such as MDA.This study showed that highly thermal oxidized frying oil feeding decreased SOD activity (2.21 unit/ml) meanwhile control group (non oxidative stress group) showed SOD activity of 4.02 unit/ml (Fig. 1).Highly thermal oxidized frying oil was a source of free radicals that caused oxidative stress indicated by declined SOD activity. Exposure to high amount of free radicals led to impairment of antioxidant defense system because antioxidant enzymes such as SOD should have to stabilize free radicals that lead to reduce their activity. Zadaket al.[32] explained that in human body there is a mechanism to overcome the damage due to ROS existence. Important enzymes to reduce oxidator level in tissues and cells are SOD, glutathione peroxidase, and catalase. Toxic free radicals are usually inactivated or scavenged by antioxidant before they destroy lipid, protein, or nucleic acid[33]. SOD converts superoxide anion into hydrogen peroxide (H2O2) and O2, and italso protects cell from endogeneous and exogeneous ROS formation, as well[34]. In the case of high free radicals, there is an imbalance between antoxidant and oxidant activities that causes oxidative stress[35]. Therefore, in this condition ingestion of antioxidant as exogeneous antioxidant will help to protect the body from oxidative stress.Zaidi and Banu[36] revealed that in oxidative stress condition, there is a decrease in SOD, glutathione-S-tranferase (GST), catalase, and gluthatione peroxidase (GPx) activities of rat brain.Yang et al.[37] explained that insufficient antioxidant enzymes for ROS detoxifying causes imbalance of antioxidant and oxidant. According to Palmieri and Blendorio[38], increasing oxidative stress indicates that antioxidant defense system is not capable to inactivate ROS due to their excessive production.
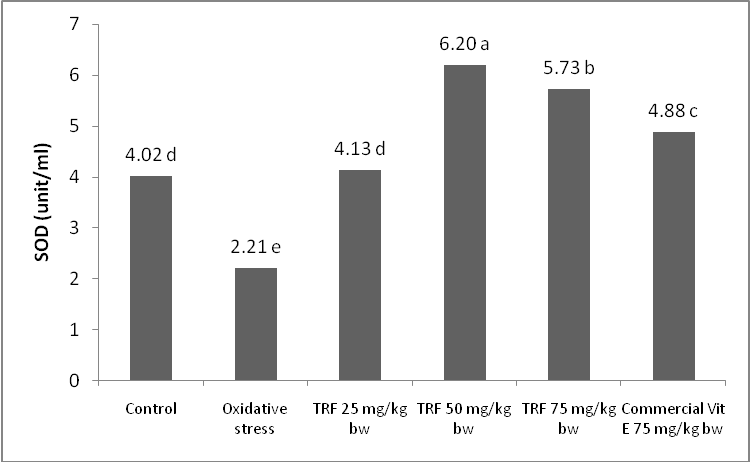 | Figure 1. SOD activity of rat groups treated by TRF dose and commercial vitamin E |
3.3. Malondialdehyde (MDA)
- Malondialdehyde (MDA) is a secondary oxidation product from lipid peroxidation, and it is a marker for lipid peroxidation related oxidative stress[41]. According to Yang et al.[37], oxidative stress is one of many cell degradation mechanisms and excessive oxidation level leads to various degenerative diseases. Free radicals attack in lipoprotein and PUFA membrane produces oxidized compounds mainly aldehydes such as malondialdehyde (MDA).Highly thermal oxidized frying oil ingestion for 4 weeks increased blood serum MDA level (74.38 ng/ml), meanwhile control group showed serum MDA level of 44.30 ng/ml (Fig. 2). Highly thermal oxidized frying oil contained free radicals that caused lipid peroxidation, indicated by MDA level increment. This MDA increase is also as an indicator for impairment of antioxidant defense system.Many studies showed that serum MDA level increased in oxidative stress and decreased by increasing antioxidant supply[37]. Antioxidant defense system is also affected by vitamin, mineral and antioxidant sufficiency from diet[32]. Suitable antioxidant supply also affects oxidant-antioxidant balance[42].
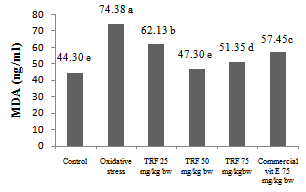 | Figure 2. MDA level of groups of rats fed by various dose of TRF and commercial vitamin E |
4. Conclusions
- The invitro antioxidant activity of TRF was affected by concentration. TRF had higher antioxidant activity than commercial vitamin E. Similarly TRF had in vivo antioxidant activity that decreased stress oxidative level and improved antioxidant status, indicated by serum SOD activity increase and serum MDA level decrease.SOD activity of groups of rats treated by TRF was higher than commercial vitamin E treated group that indicated by higher SOD activity even atlower TRF dose than that of commercial vitamin E. The SOD activity increased up to TRF dose of 50 mg/kg bw, however higher dose (75 mg/kg bw) did not showed better SOD activity. Similar result was found for MDA level. The lowest MDA level was obtained at TRF dose of 50 mg/kg bw. However, TRF dose of 75 mg/kg bw did not show lower MDA level. TRF was more effective than commercial vitamin E in decreasing MDA level. The effective dose of TRF was 50 mg/kg bw, and further increase in TRF dose did not effectively increase antioxidant activity, but possibly prooxidant.TRF was more effective antioxidant than commercial vitamin E related to tha existence of tocotrienols as the major componentof TRF.
ACKNOWLEDGMENTS
- The authors thank to Grace Martanty F. Paska and JublinaBakoil for the collaborative work in this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML