| [1] | Norman J. Temple, “Antioxidant and Disease: More Questions Than Answers”, Elsevier Ltd, Nutrition Research, vol. 30, no. 3, pp. 449-459, 2000. |
| [2] | Walter C. Willett, “Balancing Life-Style and Genomics Research for Disease Prevention”, American Association for the Advancement of Science, Science, vol. 296, no. 5568, pp. 695-698, 2002. |
| [3] | Agnieszka Szajdek, E. J. Borowska, “Bioactive Compounds and Health-Promoting Properties of Berry Fruits: a Review”, Springer, Plant Foods for Human Nutrition, vol. 63, no.4, pp. 147-156, 2008. |
| [4] | Lilian U. Thompson, “Antioxidant and Hormone-Mediated Health Benefits of Whole Grains”, Taylor & Francis, Critical Reviews in Food Science and Nutrition, vol. 34, no. 5-6, pp. 473-497, 1994. |
| [5] | Harold E. Seifried, Darrell E. Anderson, Evan I. Fischer, John A. Milner, “A Review of the Interaction among Dietary Antioxidants and Reactive Oxygen Species” Elsevier Ltd, Journal of Nutritional Biochemistry, vol. 18, no. 9, pp. 567-579, 2007. |
| [6] | Sandra Mary Lima Vasconcelos, Marília Oliveira Fonseca Goulart, José Benedito de França Moura, Vanusa Manfredini, Mara da Silveira Benfato, Lauro Tatsuo Kubota, “Reactive Oxygen and Nitrogen Species, Antioxidants and Markers of Oxidative Damage in Human Blood: Main Analytical Methods for Their Determination”, Sociedade Brasileira de Química, Química Nova, vol. 30, no. 5, pp.1323-1338, 2007. |
| [7] | Sônia Machado Rocha Ribeiro, José Humberto de Queiroz, Maria do Carmo Gouveia Pelúzo, Neuza Maria Brunoro Costa, Sérgio Luis Pinto da Matta, Maria Eliana Lopes Ribeiro de Queiroz, “The Formation and The Effects os the Reactive Oxygen Species in Biological Media”, Bioscience Journal, vol. 21, no. 3, pp. 133-149, 2005. |
| [8] | Eunok Choe, David B. Min, “Chemistry and Reactions of Reactive Oxygen Species in Foods”, Institute of Food Technologists, Journal of Food Science, vol. 70, no. 9, pp. R142-R159, 2005. |
| [9] | Rui Hai Liu, “Supplement Quick Fix Fails to Deliver”, Food Technology International, vol. 1, no. 1, pp. 71-72, 2002. |
| [10] | Michelle L. Fraser, Andy H. Lee, Colin W. Binns, “Lycopene and Prostate Cancer: Emerging Evidence”, Expert Review Anticancer Therapy, vol. 5, no. 5, pp. 847-854, 2005. |
| [11] | Wilhelm Stahl, Helmut Sies, “Bioactivity and protective effects of natural carotenoids”, Elsevier Ltd, Biochimica et Biophysica Acta - Molecular Basis of Disease, vol. 1740, no. 2, pp. 101-107, 2005. |
| [12] | Norman I. Krinsky, John T. Landrum, Richard A. Bone, “Biologic Mechanisms of the Protective Role of Lutein and Zeaxanthin in the Eye”, Annual Reviews, Annual Review of Nutrition, vol. 23, pp. 171-201, 2003. |
| [13] | Hong Wang, Guohua Cao, Ronald L. Prior, “Total Antioxidant Capacity of Fruits”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 44, no. 3, pp. 701-705, 1996. |
| [14] | Navindra P. Seeram, Muraleedharan Nair, “Inhibition of Lipid Peroxidation and Structure-Activity-Related Studies of the Dietary Constituents Anthocyanins, Anthocyanidins, and Catechins”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 50, no. 19, pp. 5308-5312, 2002. |
| [15] | Michael Heinrich, Tasleem Dhanji, Ivan Casselman, “Açai (Euterpe oleracea Mart.) – A Phytochemical and Pharmacological Assessment of the Species Health Claims”, Elsevier Ltda, Phytochemistry Letters, vol. 4, no.1, pp. 10-21, 2011. |
| [16] | Jie Kang, Keshari M. Thakali, Chenghui Xie, Miwako Kondo, Yudong Tong, Boxin Ou, Gitte Jensen, Marjorie B. Medina, Alexander G. Schauss, Xianli Wu, “Bioactivities of Açaí (Euterpe precatoria Mart.) Fruit Pulp, Superior Antioxidant and Anti-Inflammatory Properties to Euterpe olerace Mart.”, Elsevier Ltd, Food Chemistry, vol. 133, no. 3, pp. 671-677, 2012. |
| [17] | Mst. Sorifa Akter, Sejong Oh, Jong-Bang Eun, Maruf Ahmed, “Nutritional Compositions and Health Promoting Phytochemicals of Camu-Camu (Myrciaria dubia) fruit: A Review”, Elsevier Ltd, Food Research International, vol. 44, no. 7, pp. 1728-1732, 2011. |
| [18] | Maria I. Genovese, Maria da Silva Pinto, Any Elisa de Souza Schmidt Gonçalvez, Franco Maria Lajolo, “ Bioactive Compounds and Antioxidant Capacity of Exotic Fruits and Commercial Frozen Pulps from Brazil”, Sage Publications, Food Science and Technology International, vol. 14, no. 3, pp.207-214, 2008. |
| [19] | Maria do Socorro M. Rufino, Ricardo E. Alves, Edy S. de Brito, Jara Pérez-Jiménez, Fulgêncio Saura-Calixto, Jorge Mancini-Filho, “Bioactive Compounds and Antioxidant Capacities of 18 non-traditional tropical fruits from Brazil”, Elsevier Ltd, Food Chemistry, vol. 121, no. 4, pp. 996-1002, 2010. |
| [20] | Renan Campos Chisté, Marisa Freitas, Adriana Zerlotti Mercadante, Eduarda Fernandes, “The Potential of Extracts of Caryocar villosum Pulp to Scavenge Reactive Oxygen and Nitrogen Species”, Elsevier Ltd, Food Chemsitry, vol. 135, no. 3, pp. 1740-1749, 2012. |
| [21] | Online Available: http://www.biomasdobrasil.com/ |
| [22] | Roberta Cunha de Mendonça, Jeanine Maria Felfini, Bruno Machado Teles Walter, Manoel Claudio da Silva, Alba Valéria Rezende, Tarciso S. Filgueiras, Paulo Ernane Nogueira, “Flora Vascular do Bioma Cerrado” in S. M. Sano, S. P. Almeida (Eds.), Cerrado, Ambiente e Flora, Embrapa, Brazil, pp. 289-556, 1998. |
| [23] | Carolyn Proença, Rafael S. Oliveira, Ana Palmira Silva, “Flores e Frutas do Cerrado”, Editora Rede Sementes do Cerrado, 2ª ed, Brazil, 2006. |
| [24] | J. A. Ratter, J. F. Ribeiro, S. Bridgewater, “The Brazilian Cerrado Vegetation and Threats to its Biodiversity”, Oxford Journals, Annals of Botany, vol. 80, no. 3, pp. 223-230, 1997. |
| [25] | A. K. Glyan’ko, G. G. Vasil’eva, “Reactive Oxygen and Nitrogen Species in Legume-Rhizobial Symbiosis: A review”, Springer, Applied Biochemistry and Microbiology, vol. 46, no. 1, pp. 15-22, 2010. |
| [26] | Barry Halliwell, John M. C. Gutteridge, “Free Radicals in Biology and Medicine”, OUP Oxford, 4a ed., United States, pp. 280-525, 2007. |
| [27] | Marian Valko, Dieter Leibfritz, Jan Moncol, Mark T. D. Cronin, Milan Mazur, Joshua Telser, “Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease”, Elsevier Ltd, International Journal of Biochemistry e Cell Biology, vol. 39, no. 1, pp. 44-84. |
| [28] | Christine C. Winterbourn, “Reconciling the Chemsitry and Biology of Reactive Oxygen Species”, Nature Publishing Group, Nature Chemical Biology, vol. 4, no 5, pp. 278-286, 2008. |
| [29] | Marian Valko, C. J. Rhodes, Jan Moncol, M. Izakovic, Milan Mazur, “Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer”, Elsevier Ltd, Chemico_Biological Interactions, vol. 160, no. 1, pp. 1-40, 2006. |
| [30] | Dejian Huang, Boxin Ou, Ronald L. Prior, “The Chemistry Behind Antioxidant Capacity Assays”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 53, no. 6, pp. 1841-1856. |
| [31] | Marjolaine Roche, Claire Dufour, Nathalie Mora, Olivier Dangles, “Antioxidant Activity of Olive Phenols: Mechanistic Investigation and Characterization of Oxidation Products by Mass Spectroscopy”, The Royal Society of Chemistry, Organic & Biomolecular Chemistry, vol. 03, pp. 423-430, 2005. |
| [32] | Paolo Di Mascio, Stephan Kaiser, Helmut Sies, “Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher”, Elsevier Ltd, Archives of Biochemistry and Biophysics, vol. 274, no. 2, pp. 532-538, 1989. |
| [33] | Mariana A. Montenegro, Alessandro de Oliveira Rios, Adriana Zerlotti Mercadante, Mônica A. Nazareno, Claudio Dario Borsarelli, “Model Studies on the Photosensitized Isomerization of Bixin” Americal Chemical Society, Journal of Agricultural and Food Chemistry, vol. 52, no. 2, pp.367-373, 2004. |
| [34] | Semih Otles, Ozlen Çagindi, “Carotenoids as Natural Colorants” in Carmen Socaciu (Ed.), Food Colorants: Chemical and Functional Properties, CRC Press, United States, pp. 51-70, 2007 |
| [35] | George Britton, Synn Liaaen-Jensen, Hanspeter Pfander, Carotenoids Handbook. Birkhäuser, Switzerland, 2004. |
| [36] | Ali El-Agamey, Gordon M. Lowe, David J. McGarvey, Alan Mortensen, Denise M. Phillip, T. George Truscott, Andrew J. Young, “Carotenoid Radical Chemistry and Antioxidant/Pro-oxidant Properties, Elsevier Ltd, Archives of Biochemistry and Biophysics, vol. 430, no. 1, pp. 37-48, 2004. |
| [37] | Jian-Jhih Guo, Ching-Han Hu, “Mechanism of Chain Termination in Lipid Peroxidation by Carotenes: a Theoretical Study”, American Chemical Society, The Journal of Physical Chemistry B, vol. 114, no. 50, pp. 16948-16958, 2010. |
| [38] | Wilfred Vermerris, Ralph Nicholson, Phenolic Compound Biochemistry, Springer Science + Business Media BV, United States, 2008. |
| [39] | Marian Naczk, Fereidoon Shahidi, “Extraction and Analysis of Phenolic in Food”, Elsevier Ltd, Journal of Chromatography A, vol. 1054, no. 1-2, pp. 95-111, 2004. |
| [40] | Ock Kyoung Chun, Sang Jin Chung,Won O. Song, “Estimated Dietary Flavonoid Intake and Major Food Sources of U.S. Adults”, American Society for Nutrition, The Journal of Nutrition, vol. 137, no. 5, pp. 1244-1252, 2007. |
| [41] | Paola R. Arabbi, Maria Inês Genovese, Franco Maria Lajolo, “Flavonoids in Vegetable Foods Commonly Consumed in Brazil and Estimated Ingestion by the Brasilian Population”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 52, no. 5, pp. 1124-1131, 2004. |
| [42] | Obdúlio Benavente-García, Julían Castillo, Francisco R. Marin, Ana Ortuno, José A. Del Rio, “Uses and Properties of Citrus Flavonoids”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 45, no. 12, pp. 4505-4515, 1997. |
| [43] | Øyvind M. Andersen, Kenneth R. Markham, “Flavonoids: Chemistry, Biochemistry and Applications”, CRC Press, United States, 2005. |
| [44] | Alan Crozier, Indu B. Jaganath, Michael N. Clifford, “Dietary Phenolics: Chemistry, Bioavailability and Effects on Health”, Royal Society of Chemistry, Natural Products Report, vol. 26, no. 8, pp. 1001-1043, 2009 |
| [45] | P. C. H. Hollman, M. B. Katan, “Dietary flavonoids: intake, health effects and bioavailability”, Elsevier, Food and Chemical Toxicology, vol. 37, no. 9-10, pp. 937-942, 1999. |
| [46] | Fujiki Hirota, Kazue Imai, Kei Nakachi, Masahita Shimizu, Hisataka Moriwaki, Massami Suganuma, “Challenging the Effectiveness of Green Tea in Primary and Tertiary Cancer Prevention”, Springer, Journal of Cancer Research Clinical Oncology, vol. 138, no. 8, pp. 1259-1270, 2012. |
| [47] | Jurgen F. Leikert, Thomas R. Räthel, Paulus Wohlfart, Véronique Cheynier, Angélika M. Vollmar, Verena M. Dirsch, “Red Wine Polyphenols Enhance Endothelial Nitric Oxide Synthase Expression and Subsequent Nitric Oxide Release From Endothelial Cells, American Heart Association, Circulation, vol. 106, no. 13, pp. 1614-1617, 2012. |
| [48] | Volker Spitzer, Florian Schweigert, “Vitamin Basic, The Facts about Vitamins in Nutrition”, 3a Ed, DSM Nutritional Products Ltd, Germany, 2007. |
| [49] | Andre Theriault, Jun-Tzu Chao, Qi Wang, Adbul Gapor, Khosrow Adeli, “Tocotrienol: a Review of its Therapeutic Potential”, Elsevier, Clinical Biochemistry, vol. 32, no. 5, pp. 309-319, 1999. |
| [50] | Francesca Mangialasche, Weili Xu, Miia Kivipelto, Emanuela Costanzi, Sara Ercolani, Martina Pigliautile, Roberta Cecchetti, Mauro Baglioni, Andrew Simmons, Hilkka Soininen, Magda Tsolaki, Iwona Kloszewska, Bruno Vellas, Simon Lovestone, Patrizia Mecocci, “Tocopherols and Tocotrienols Plasma Levels are Associated with Cognitive Impairment”, Elsevier, Neurobiology of Aging, vol. 33, no. 10, pp. 2282-2290, 2012. |
| [51] | Hong Wang, Guohua Cao, Ronald L. Prior, “Oxygen Radical Absorbing Capacity of Anthocyanins”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 45, no. 2, pp. 304-309, 1997. |
| [52] | Anna R. Proteggente, Ananth Sekher Pannala, George Paganga, Leo van Buren, Eveline Wagner, Sheila Wiseman, Frans van de Put, Clive Dacombe, Catherine A. Rice-Evans, “The Antioxidant Activity of Regularly Consumed Fruit and Vegetable Reflects Their Phenolic and Vitamin C Composition”, Free Radical Research, vol. 36, no. 2, pp. 217-233, 2002. |
| [53] | Joe A. Vinson, Xuehui Su, Ligia Zubik, Pratima Bose, “Phenol Antioxidant Quantity and Quality in Foods: Fruits”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 49, no. 11, pp. 5315-5321, 2001. |
| [54] | Reşat Apak , Kubilay Güçlü , Mustafa Özyürek , Saliha Esin Karademir, “Novel Total Antioxidant Capacity Index for Dietary Polyphenols, Vitamins C and E, using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method” American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 52, no. 26, pp. 7970-7981, 2004. |
| [55] | Reşat Apak , Kubilay Güçlü , Mustafa Özyürek , Saliha Esin Karademir, Mehmet Altun, “Total Antioxidant Capacity Assay of Human Serum Using Copper(II)-neocuproine as Chromogenic Oxidant: The CUPRAC Method”, Free Radical Research, vol. 39, no. 9, pp. 949-961, 2005. |
| [56] | Jie Sun, Yi-Fang Chu, Xianzhong Wu, Rui Hai Liu, “Antioxidant and Antiproliferative Activities of Common Fruits”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 50, no. 25, pp. 7449-7954, 2002. |
| [57] | Bente L. Halvorsen, Kari Holte, Mari C. Myhrstad, Ingrid Barikmo, Erlend Hvattum, Siv Fagertun Remberg, Anne-Brit Wold, Karin Haffner, Halvard Baugerod, Lene Frost Andersen, Ø Moskaug, David Jacobs, Rune Blomhoff, “A Systematic Screening of Total Antioxidants in Dietary in Plants”, American Society of Nutrition, The Journal of Nutrition, vol. 132, no. 3, pp.461-471, 2002. |
| [58] | Nicoletta Pellegrini, Mauro Serafini, Barbara Colombi, Daniele Del Rio, Sara Salvatore, Marta Bianchi, Furio Brighenti, “Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in Vitro Assays”, American Society of Nutrition, The Journal of Nutrition, vol. 133, no. 9, pp. 2812-281, 2003. |
| [59] | Roberta Re, Nicoletta Pellegrini, Anna Proteggente, Ananth Pannala, Min Yang, Catharine Rice-Evans, “Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay”, Elsevier Ltd, Free Radical Biology and Medicine, vol. 26, no. 9, pp. 1231-1237, 1999. |
| [60] | Reşat Apak , Esma Tütem, Mustafa Özyürek, Kubilay Güçlü , “Antioxidant Activity/Capacity Assay Methods Applied to Fruit and Cereals” in Fruit and Cereal Bioactives – Sources, Chemistry, and Applications, CRC Press, United States, 2011. |
| [61] | Guohua Cao, C. P. Verdon, H. A. Wu, Hong Wang, Ronald L. Prior, “Automated Assay of Oxygen Radical Absorbance Capacity with the COBAS FARA II ”, American Association for Clinical Chemistry, Clinical Chemistry, vol. 41, no. 12, pp. 1738-1744, 2005. |
| [62] | Boxin Ou, Maureen Hampsch-Woodil, Ronald L. Prior, “Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 49, no. 10, pp. 4619-4626, 2001. |
| [63] | Ronald L. Prior, Ha Hoang, Liwei Gu, Xianli Wu, Mara Bacchiocca, Luke Howard, Maureen Hampsch-Woodill, Dejian Huang, Boxin Ou, Robert Jacob, “Assays for Hydrophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORAC(FL))) of Plasma and other Biological and Food Samples”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 51, no. 11, pp. 3273-3279, 2003. |
| [64] | Nicholas J. Miller, Catherine A. Rice-Evans, M. J. Davies, V. Gopinathan, A. Wilner, “A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates”, Clinical Science, vol. 84, no. 4, pp. 407-412, 1993. |
| [65] | Catherine A. Rice-Evans, Nicholas J. Miller, George Paganga, “Structure Antioxidant Activity Relationships of Flavonoids and Phenolic Acids”, Elsevier, Free Radical Biology and Medicine, vol. 20, no. 7, pp.933-956, 1996. |
| [66] | Vitaly Roginski, Eduardo A. Lissi, “Review of Methods for Determine Chain-Breaking Antioxidant Activity in Food”, Elsevier, Food Chemistry, vol. 92, no. 2, pp. 235-254, 2005. |
| [67] | W. Brand-Willians, M. E. Cuvelier, C. Berset, “Use of a Free Radical Method to Evaluate Antioxidant Activity”, Elsevier, LWT-Food Science and Technology, vol. 28, no. 1, pp. 25-30, 1995. |
| [68] | Iris F. F. Benzie, J. J. Strain, “The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘‘Antioxidant Power’’: The FRAP Assay”, Academic Press Inc, Analytical Biochemistry, vol. 239, no. 1, pp. 70-76, 1996. |
| [69] | Lisbeth A. Pacheco-Palencia, Christopher E. Duncan and Stephen T. Talcott, “Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria”, Elsevier, Food Chemistry, vol. 115, no. 4, pp. 1199–1205, 2009. |
| [70] | Veridiana Vera De Rosso, Silke Hillebrand, Elyana Cuevas Montilla, Florianda O. Bobbio, Peter Winterhalter, Adriana Zerlotti Mercadante, “Determination of Anthocyanins from Acerola (Malpighia emarginata DC.) and Açai (Euterpe oleracea Mart.) by HPLC-PDA and HPLC-MS, Elsevier, Journal of Food Composition and Analysis, vol. 21, no. 4, pp. 291-299, 2008. |
| [71] | Juliana Carvalho Ribeiro, Lusânia Maria Greggi Antunes, Alexandre Ferro Aissa, Joana D’arc Castania Darin, Veridiana Vera De Rosso, Adriana Zerlotti Mercadante, Maria de Lourdes Pires Bianchi, “Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay”, Elsevier Ltd, Mutation Research. Genetic Toxicology and Environmental Mutagenesis, vol. 695, no. 1-2, p. 22-28, 2010. |
| [72] | André Gordon, Ana Paula Gil Cruz, Lourdes Maria Corrêa Cabral, Sidinéa Cordeiro de Freitas, Cristina Maria Araujo Dib Taxi, Carmen Marino Donangelo, Rafaella de Andrade Mattietto, Mirko Friedrich, Virgínia Martins da Matta, Friedhelm Marx, “Chemical Characterization and Evaluation of Antioxidant Properties of Açaí fruits (Euterpe oleraceae Mart.) During Ripening”, Elsevier, Food Chemistry, vol. 133, no. 2, pp. 256-263, 2012. |
| [73] | Melina Oliveira de Souza, Maísa Silva, Marcelo Eustáquio Silva, Riva de Paula Oliveira, Maria Lucia Pedrosa, “Diet Supplementation with Acai (Euterpe oleracea Mart.) Pulp Improves Biomarkers of Oxidative Stress and the Serum Lipid Profile in Rats”, Elsevier, Nutrition, vol. 26, no. 7-8, pp. 804-810, 2010. |
| [74] | Gitte S. Jensen, Xianli Wu, Kelly M. Patterson, Janelle Barnes, Steve G. Carter, Larry Scherwitz, Robert Beamam, John R. Endres, Alexandre Schauss, “In Vitro and in Vivo Antioxidant and Anti-Inflammatory Capacities of an Antioxidant-Rich Fruit and Berry Juice Blend. Results of a Pilot and Randomized, Double-Blinded, Placebo-Controlled, Crossover Study”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 56, no. 18, pp. 8326-8333, 2008. |
| [75] | Cinthia Fernanda Zanatta, Elyana Cuevas, Florinda O. Bobbio, Peter Winterhalter, Adriana Zerlotti Mercadante, “Determination of Anthocyanins from Camu-Camu (Myrciaria dubia) by HPLC-PDA, HPLC-MS, and NMR”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 53, no. 24, pp. 9531-9535, 2005. |
| [76] | Cinthia Fernanda Zanatta, Adriana Zerlotti Mercadante, “Carotenoid Composition from the Brazilian Tropical Fruit Camu-Camu (Myrciaria dubia)”, Elsevier, Food Chemistry, vol. 101, no. 4, pp. 1526-1532, 2007. |
| [77] | Rosana Chirinos, Jorge Galarza, Indira Betalleluz-Pallardel, Romina Pedreschi, David Campos, “Antioxidant Compounds and antioxidant Capacity of Peruvian Camu Camu (Myrciaria dubia (H.B.K.) McVaugh) Fruit at Different Maturity Stages”, Elsevier, Food Chemistry, vol. 120, no. 4, pp. 1019-1024, 2010. |
| [78] | Ricardo Elesbão Alves, Heloisa Almeida Cunha Filgueiras, Carlos Farley Hebster Moura, Nágela Cristina Costa Araújo, Adriano Silva Almeida, “Camu-camu (Myrciaria dubia McVaugh): A Rich Natural Source of Vitamin C”, in Proceedings of the InterAmericam Society for Tropical Horticulture”, vol. 46, pp. 11-13, 2002. |
| [79] | Kurt A. Reynertson, Hui Yang, Bei Jiang, Margaret J. Basile, Edward J. Kennelly, “Quantitative Analysis of Antiradical Phenolic Constituents from Fourteen Edible Myrtaceae Fruits”, Elsevier, Food Chemistry, vol. 109, no. 4, pp. 883-890, 2008. |
| [80] | Any Elisa De Souza Schmidt Gonçalves, Franco Maria Lajolo, Maria Inês Genovese, “Chemical Composition and Antioxidant/Antidiabetic Potential of Brazilian Native Fruits and Commercial Pulps”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 58, no. 8, pp. 4666-4674, 2010. |
| [81] | Roberta Belandrino Rodrigues, Menelaos Papagiannopoulos, José Guilherme Soares Maia, Kaoru Yuyama, Friedhelm Marx, “Antioxidant Capacity of Camu Camu (Myrciaria dubia (H.B.K.) McVaugh) Pulp”, Ernährung/Nutrition, vol. 30, no. 9, pp. 357-362, 2006. |
| [82] | Francisco Carlos da Silva, Andrelisse Arruda, Alexandre Ledel, Cíntia Dauth, Nathalia Faria Romão, Rafaele Nazário Viana, Alexandre de Barros Ferraz, Jaqueline Nascimento Picada, Patrícia Pereira, “Antigenotoxic Effect of Acute and Chronic Treatments with Amazonian Camu-Camu (Myrciaria dubia) Juice on Mice Blood Cells”, Elsevier, Food and Chemical Toxicology, vol. 50, no. 7, pp. 2275-2281, 2012. |
| [83] | Teruo Inoue, Hiroshi Komoda, Toshihiko Uchida, Koichi Node, “Tropical Fruit Camu-Camu (Myrciaria dubia) has Anti-Oxidative and Anti-Inflammatory Properties”, Elsevier, Journal of Cardiology, vol. 52, no. 2, pp. 127-132, 2008. |
| [84] | Renan Campos Chisté, Adriana Zerlotti Mercadante, “Identification and Quantification, by HPLC-DAD-MS/MS, of Carotenoids and Phenolic Compounds from the Amazonian Fruit Caryocar villosum”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 60, no. 23, pp. 5884-5892, 2012. |
| [85] | Mara Ribeiro Almeida, Joana D’Arc Castania Darin, Lívia Cristina Hernandes, Alexandre Ferro Aissa, Renan Campos Chisté, Adriana Zerlotti Mercadante, Lusânia Maria Greggi Antunes, Maria Lourdes Pires Bianchi, “Antigenotoxic Effects of Pequiá (Caryocar villosum) in Multiple Rat Organs”, Springer, Plant Foods for Human Nutrition, vol. 67, no. 2, pp. 171-177, 2012. |
| [86] | Xianli Wu, Gary R. Beecher, Joanne M. Holden, David B. Haytowitz, Susan E. Gebhardt, Ronald L. Prior, “Lipophilic and Hydrophilic Antioxidant Capacities of Common Foods in the United States”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 52, no. 12, pp. 4026-4037, 2004. |
| [87] | Jesus N. S. Souza, Evaldo M. Silva, Adeline Loir, Jean-François Rees, Hervé Hogez, Yvan Larondelle, “Antioxidant Capacity of Four Polyphenol-Rich Amazonian Plant Extracts: A Correlation Study using Chemical and Biological in Vitro Assays”, Elsevier, Food Chemistry, vol. 106, no. 1, pp. 331-339, 2008. |
| [88] | Alexandre G. Schauss, Xianli Wu, Ronald L. Prior, Boxin Ou, Dejian Huang, Jonh Owens, Amit Agarwal, Gitte S. Jensen, Aaron N. Hart, Edward Shanbrom, “Antioxidant Capacity and Other Bioactivities of the Freeze-Dried Amazonian Palm Berry, Euterpe oleracea Mart. (Acai)”, American Chemical Society, Journal of Agricultural and Food Chemistry, vol. 54, no. 22, pp. 8604-8610, 2006. |
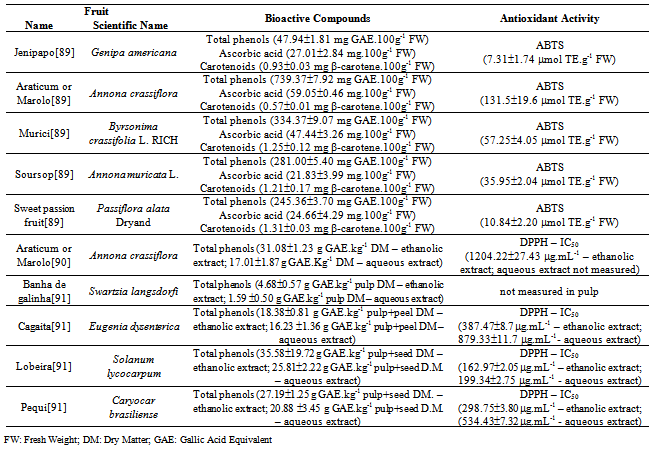
| [89] | Vanessa Rios de Souza, Patrícia Aparecida Pimenta Pereira, Fabiana Queiroz, Soraia Vilela Borges, João de Deus Souza Carneiro, “Determination of Bioactive Compounds, Antioxidant Activity and Chemical Composition of Cerrado Brazilian Fruits”, Elsevier Ltd, Food Chemistry, vol. 134, no. 1, pp. 381-386, 2012. |
| [90] | Roberta Roesler, Luciana G. Malta, Luciana C. Carrasco, Gláucia Pastore, “Evaluation of the Antioxidant Properties of the Brazilian Cerrado Fruit Annona crassiflora (Araticum)”, Institute of Food Technologists, Journal of Food Science, vol. 71, no. 2, pp. C102-C107, 2006. |
| [91] | Roberta Roesler, Luciana Gomes Malta, Luciana Cristina Carrasco, Roseane Barata Holanda, Clélia Alves Socorro Sousa, Glaucia Maria Pastore, “Atividade Antioxidante de Frutas do Cerrado” Sociedade Brasileira de Ciência e Tecnologia de Alimentos, Ciência e Tecnologia de Alimentos, vol. 27, no. 1, pp. 53-60, 2007. |
| [92] | Roberta Roesler, Rodrigo R. Catharino, Luciana G. Malta, Marcos N. Eberlin, Glaucia Maria Pastore, “Antioxidant Activity of Caryocar brasiliense (pequi) and Characterization of Components by Electrospray Ionization Mass Spectrometry”, Elsevier, Food Chemistry, vol. 110, no. 3, pp. 711-717, 2008. |
| [93] | Alessandro de Lima, Ana Mara de Oliveira e Silva, Reginaldo Almeida Trindade, Rosângela Pavan Torres, Jorge Macini-Filho, “Chemical Composition and Bioactive Compounds in the Pulp and Almond of Pequi Fruit (Caryocar brasiliense, Camb.)”, Revista Brasileira de Fruticultura, vol. 29, no. 3, pp. 695-698, 2007. |
| [94] | Cristiane H. Azevedo-Meleiro, Delia B. Rodriguez-Amaya, “Confirmation of the Identity of the Carotenoids of Tropical Fruits by HPLC-DAD and HPLC-MS”, Elsevier, Journal of Food Composition and Analysis, vol. 17, no. 3-4, pp. 385-396, 2004. |
| [95] | Ana L. Miranda-Vilela, Inês S. Resck, Cesar K. Grisolia, “Antigenotoxic Activity and Antioxidant Properties of Organic and Aqueous Extracts of Pequi Fruit (Caryocar brasiliense Camb.) Pulp”, Sociedade Brasileira de Genética, Genetics and Molecular Biology, vol. 31, no. 4, pp. 956-963, 2008. |
| [96] | E.C. Aguilar, T.L. Jascolka, L.G. Teixeira, P.C. Lages, A.C.C. Ribeiro, E.L.M. Vieira, M.C.G. Peluzio, J.I. Alvarez-Leite, “Paradoxical Effect of a Pequi Oil-Rich Diet on the Development of Atherosclerosis: balance Between Antioxidant and Hyperlipidemic Properties”, Brazilian Journal of Medical and Biological Research, vol. 45, pp. 601-609, 2012. |
| [97] | Lucile Abe, Franco M. Lajolo, Maria Inês Genovese, “Potential Dietary Sources of Ellagic Acid and other Antioxidants Among Fruits Consumed in Brazil: Jabuticaba (Myrciaria jaboticaba (Vell.) Berg)”, Society of Chemical Industry, Journal of Science Food and Agriculture, vol. 92, no. 8, pp.1679-1687, 2012. |
| [98] | Shi-Biao Wu, Keyvan Dastmalchi, Chunlin Long, Edward J. Kennelly, “Metabolite Profiling of Jaboticaba (Myrciaria cauliflora) and other Dark-Colored Fruit Juices”, American Chemical Society Publications, Journal of Agricultural and Food Chemistry, vol. 60, no. 30, pp. 7513-7525, 2012. |
| [99] | Diego T. Santos, Priscilla C. Veggi, M. Angela A. Meireles, “Extraction of Antioxidant Compounds from Jabuticaba (Myrciaria cauliflora) skins: Yield, Composition and Economical Evaluation”, Elsevier Ltd, Journal of Food Engineering, vol. 101, no. 1, pp. 23-31, 2010. |
| [100] | Giovana Bonat Celli, Adaucto Bellarmino Pereira-Neto, Trust Beta, “Comparative Analysis of Total Phenolic Content, Antioxidant Activity, and Flavonoids Profile of Fruits from Two Varieties of Brazilian Cherry (Eugenia uniflora L.) Throughout the Fruit Development Stages”, Elsevier, Food Research International, vol. 44, no. 8, pp. 2442-2451, 2011. |
| [101] | Milena Bagetti, Elizete Maria Pesamosca Facco, Jaqueline Piccolo, Gabriela Elisa Hirsch, Delia Rodriguez-Amaya, Cintia Nanci Kobori, Márcia Vizzotto, Tatiana Emanuelli, “Physicochemical Characterization and Antioxidant Capacity of Pitanga Fruits (Eugenia uniflora L.)”, Sociedade Brasileira de Ciência e Tecnologia de Alimentos, Ciência e Tecnologia de Alimentos, vol. 31, no. 1, pp.147-154, 2011. |
| [102] | Genival Lopes Filho, Veridiana Vera De Rosso, Maria Angela A. Meireles, Paulo de Tarso da Rosa, Alexandra L. Oliveira, Adriana Z. Mercadante, Fernando A Cabral, “Supercritical CO2 Extraction of Carotenoids from Pitanga Fruits (Eugenia uniflora L.)”, Elsevier, The Journal of Supercritical Fluids, vol. 46, no. 1, pp. 33-39, 2008. |
| [103] | Edy Sousa De Brito, Manuela Cristina Pessanha de Araújo, Ricardo Elesbão Alves, Colleen Carkeet, Beverly A. Clevidence, Janet A. Novotny, “Anthocyanins Present in Selected Tropical Fruits: Acerola, Jambolão, Jussara, and Guajiru”, American Chemical Society Publications, Journal of Agricultural and Food Chemistry, vol. 55, no. 23, pp. 9389-9394, 2007. |
| [104] | Graciele da Silva Campelo Borges, Francilene Gracieli Kunradi Vieira, Cristiane Copetti, Luciano Valdemiro Gonzaga, Rui Carlos Zambiazi, Jorge Macini Filho, Roseane Fett, “Chemical Characterization, Bioactive Compounds, and Antioxidant Capacity of Jussara (Euterpe edulis) Fruit from the Atlantic Forest in Southern Brazil”, Elsevier, Food Research International, vol. 44, no. 7, pp. 2128-2133, 2011. |
| [105] | Marina C. Pereira, Rosana S. Steffens, André Jablonski, Plinho F. Hertz, Alessandro de O. Rios, Márcia Vizzoto, Simone H. Flores, “Characterization and Antioxidant Potential of Brazilian Fruits from the Myrtaceae Family”, American Chemical Society Publications, Journal of Agricultural and Food Chemistry, vol. 60, no. 12, pp. 3061-3067, 2012. |
| [106] | Adelia F. Faria, Marcella C. Marques, Adriana Z. Mercadante, “Identification of Bioactive Compounds from Jambolão (Syzygium cumini) and Antioxidant Capacity Evaluation in Different pH Conditions”, Elsevier, Food Chemistry, vol. 126, no. 4, pp. 1571-1578, 2011. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML