-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(1): 28-36
doi:10.5923/j.fph.20130301.04
The Existing Studies on Biosynthesis of Poly(ɣ-glutamic acid) by Fermentation
Luana P. Moraes , Priscila N. Brito , Ranulfo M. Alegre
Laboratory of Fermentation Processes and Wastewater Treatment/Dea/Fea (School of Food Engineering)/Unicamp (University of Campinas) Cidade Universitária "Zeferino Vaz", R. Monteiro Lobato, 80; 13083-862, Campinas, SP, Brazil
Correspondence to: Luana P. Moraes , Laboratory of Fermentation Processes and Wastewater Treatment/Dea/Fea (School of Food Engineering)/Unicamp (University of Campinas) Cidade Universitária "Zeferino Vaz", R. Monteiro Lobato, 80; 13083-862, Campinas, SP, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Poly(ɣ-glutamic acid) known as ɣ-PGA, is a polymer that releases the nonessential amino acid glutamic acid upon hydrolysis. The application of poly(ɣ-glutamic acid) has increased in past year, as has the research to optimise its production methods with less-expensive processes, for example, the use of new substrate carbon and nitrogen sources for the fermentation processes. The following work reports the study of different fermentation processes for ɣ-PGA production and their implementation with regard to the fermentation and nutritional requirements of the studied microorganisms.
Keywords: Poly(ɣ-glutamic acid), Fermentation, Bacillus, Biosynthesis, Nutritional Requirements
Cite this paper: Luana P. Moraes , Priscila N. Brito , Ranulfo M. Alegre , The Existing Studies on Biosynthesis of Poly(ɣ-glutamic acid) by Fermentation, Food and Public Health, Vol. 3 No. 1, 2013, pp. 28-36. doi: 10.5923/j.fph.20130301.04.
Article Outline
1. Introduction
- Several authors report that Ivánovics et al. (1937) discovered ɣ-PGA as the component of the Bacillus anthracis capsule[1-4]. Later, ɣ-PGA was identified in other Bacillus species, and Bovarnick showed in 1942 that ɣ-PGA was produced in a culture broth by Bacillus subtilis as a submerged fermentation (Smf) and solid-state fermentation (SSF) product[5]; the polymer has been well studied since.Although biopolymers formed by the union of amino acids are very similar to proteins, not being proteins, they do not have a specific sequence. Rather, they comprise a single amino acid that form polymers having molecular masses as widely diverse as those of polysaccharides[6]. These polymers can be produced by fermentation. For example, ɣ-PGA is present in natto, a traditional Japanese food prepared using soybeans fermented by Bacillus strains; the ɣ-PGA and polysaccharides are found on the bean surface[6]. The ɣ-PGA from natto consists of 50 - 80 % D- and 20 - 50 % L-glutamate as the main component of the viscous extracellular (bacterial) materials[7],[8]. The natto contains polysaccharide-containing mucin (levan-form fructan) in addition to ɣ-PGA[3],[6],[9-11], and the consumption of natto is associated with reduced bone loss in postmenopausal Japanese women[12].The aim of this review is discuss several of the recent developments in the application of Bacillus species for the production of ɣ-PGA. ɣ-PGA is biopolymer anionic, biodegradable, water-soluble with different applications and has effect on human health. In this review, the various works are briefly described. The main features and mechanisms of the ɣ-PGA synthesis and the results are discussed.
2. The ɣ-PGA Synthesis
- ɣ-PGA is synthesised by Gram-positive bacteria[13] and is produced as a polymer outside of the cell[4],[5],[14] by several Bacillus species, particularly wild-type isolates, including Bacillus subtilis IFO3335[3], Bacilluslicheniformis, Bacillus megaterium, Bacillus amyloliquefaciens[5], [15],[16] and a few other organisms[17],[18]. However, problems have been reported in the ɣ-PGA production by Bacillus licheniformis 9945a: after repeated cultivation, the strain degenerates to a non-PGA-producing strain, causing different results in the ɣ-PGA productivity and fermentation kinetics[16],[19]. Birrer et al. solved this problem in 1994 using cryogenically frozen vegetative cells[20]. The biosynthesis of ɣ-PGA in bacteria is performed in two steps: in the first step, L- and D- glutamic acid are synthesised, and these glutamic acid units are joined together in the second step[1],[21].The units of D- and L- glutamic acid are connected by amide linkages between the α-amino and ɣ-carboxylic acid[2],[4],[6],[10],[11],[22-25]. The ɣ-PGA polymer can be characterised by its molecular mass and the relationship between the D- and L-glutamic acid monomers and the carboxyl group (α- or ɣ-) connected to the peptide[26]. The structural framework is shown in Figure 1.
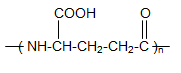 | Figure 1. Structure of ɣ-PGA[3] |
3. Enzymes Involved in ɣ-PGA Production
- Several studies have been conducted to identify the enzymes of the ɣ-PGA biochemical pathway to increase productivity[24], and reports have shown that the key step is the production of 2-oxogluturate, the precursor of L-glutamic acid[36], which is synthesised via glycolysis and the tricarboxylic acid cycle[24]. A transglutaminase has been reported to be involved in ɣ-PGA production in Bacillus subtilis NR-1[39], however transglutaminase involvement has not been found in some other works[6]. Glutamate racemase is an important enzyme in ɣ-PGA synthesis, as it is involved in the production of the substrate, more specifically in the supply of D-glutamate[32].In ɣ-PGA producing Bacillus subtilis, the production of abundant DL-glutamate occurs via glutamate racemase[22].Figure 2 shows ɣ-PGA production, whereby L-glutamic acid is produced from citric acid, which reacts to form isocitric acid; isocitric acid then reacts to form the α-ketoglutarate of the tricarboxylic acid cycle (TCA). Thus, glutamic acid is used to form ɣ-PGA, and a large amount of the polymer is produced from citric acid and ammonium sulphate[6].The pgsBCA genes are responsible for the synthesis of ɣ-PGA, with the PgsBCA system encoding the sole catalytic activity for ɣ-PGA synthesis. The polymerisation reaction in vitro is given by (1)[40]:
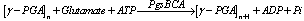 | (1) |
4. Nutritional Requirements for Different Bacillus Species
- Several parameters that affect the ɣ-PGA yield and molecular weight have been investigated, for example, the composition of the culture medium, Bacillus species, fermentation conditions, viscosity and mode of reactor feeding[37]. Additionally, owing to the presence of the enzymes that catalyse the in situ depolymerisation of ɣ-PGA, polyglutamyl hydrolase (PGA hydrolase), the final molecular weight of ɣ-PGA can decrease as the fermentation time increases[9].
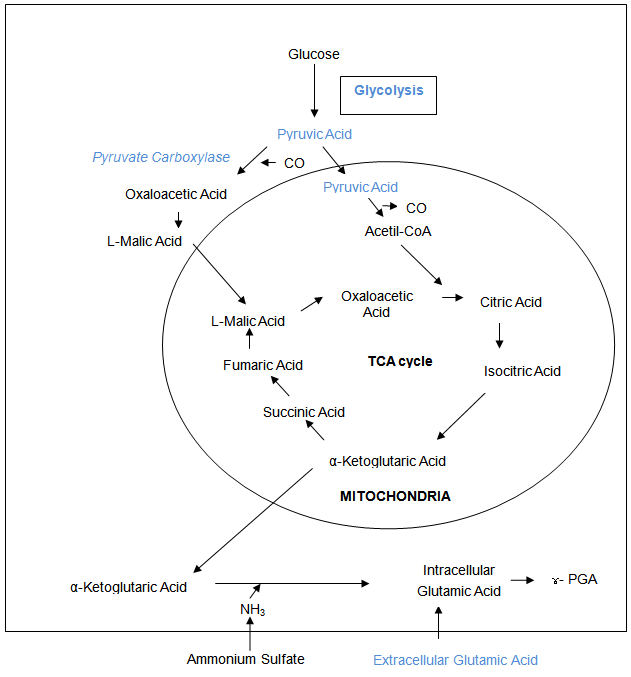 | Figure 2. Biochemical pathway of ɣ-PGA production and some modifications[3] |
5. Characteristics and Possible Applications
- ɣ-PGA is an anionic, biodegradable, water-soluble biopolymer[2],[21],[25],[45],[46] that is edible and non-toxic to humans or the environment[4],[37],[38],[45]. Its characteristics enable wide applications as a biodegradable thickener, humectant, sustained-release material, chemical vehicle in the food, cosmetic, medicine [21],[43] and pharmaceutical industries[24], cryoprotectant, curable biological adhesive, biodegradable fibre and biopolymer flocculant[4],[21]. ɣ-PGA has been suggested for medical applications in addition to drug delivery, namely as a biological glue[47],[48]. It is important to note that the ɣ-PGA polymers with different stereochemical composition have different applications: ɣ-PGA with a high content of L-glutamate is used for cosmetics due to its skin compatibility, and ɣ-PGA with a high content of D-glutamate is used because it degrades more slowly[49]. ɣ-PGA and its derivatives can be used as potential substitutes for petroleum-based hydrogels and thermoplastic polymers because it is highly water absorbent and biodegradable[50]. There are many studies concerned with modifying the chemical reactions with ɣ-PGA for the production of hydrogels and thermoplastic polymers. For example, the esterification of the carboxyl groups of ɣ-PGA produced esters that are thermoplastic[8], and a ɣ-PGA hydrogel has been produced through the ɣ-irradiation of aqueous solutions[50].Various chemically modified derivatives have been developed for the extension of the polymer utility as an environmentally important substitute for hydrogels or non-biodegradable thermoplastics[7],[50].Another interesting application was proposed by Hoppensack et al. who studied the conversion of the nitrogen content in liquid manure into biomass and ɣ-PGA by the newly isolated Bacillus licheniformis. These authors observed that, when the extensive spreading of liquid manure onto agricultural fields occurred, eutrophication of the ground and surface water and pollution of the atmosphere also occurred due to the high ammonium nitrogen content. The proposed solution was the production of biomass and ɣ-PGA in batch cultivations with swine manure and an optimised mineral salt medium using Bacillus licheniformis because of its ability to utilise the nitrogen source. For example, a reduction of the ammonium content in the liquid manure from 2.83 to 0.1 g/l and the production of 0.16 g/l ɣ-PGA and 7.5 g cell dry mass/l were observed within 410 h. In this case, the liquid manure was modified by adding 18 g citrate and 80 g glycerol/l, producing a carbon to nitrogen ratio of 15.5:1. Approximately 33 % (w/v) of the original ammonium was lost by stripping[43].The production of ɣ-PGA by Bacillus subtilis ZJU-7 isolated from fermented bean curd, a traditional Chinese food, was reported by Shi et al. in 1996. The production reached 58.2 g/l in a culture medium containing 81.05 g/l L-glutamic acid, 59.8 g/l sucrose and 53.54 g/l tryptone at 37 °C for 24 h[10].Published data showed that the ɣ-PGA production by Bacillus licheniformis 9945a is affected by the concentrations of salt, glycerol, citric acid and L-glutamic acid and the ions Mn+2 and Ca+2. The production reached 15.4 g/l in a culture medium containing 20 g/l L-glutamic acid, 12 g/l citric acid and 80 g/l glycerol, pH adjusted to 7.4 with NaOH, with the fermentation occurring for 2 - 3 days[24].Goto and Kunioka (1992) studied the biosynthesis and hydrolysis of ɣ-PGA using Bacillus subtilis IFO 3335, a strain that requires biotin as a nutrient, and observed that there was no production of ɣ-PGA in a medium without L-glutamic acid. By adding citric acid as the carbon source in a medium containing L-glutamic acid and ammonium sulphate, a large amount of ɣ-PGA was produced (9.6 g/l) without the appearance of by-products, whereas a by-product that appeared to be a polysaccharide was generated when glucose was used as the carbon source in a medium with glutamic acid. When it is not added carbon source, but using L-malic acid, succinic acid or fumaric acid in the culture medium with glutamic acid, the ɣ-PGA was hardly produced and by-product predominated. It was reported that the cells did not grow when using acetic acid as the carbon source in a culture medium with glutamic acid[3].
|
|
- After the production of ɣ-PGA, they studied its hydrolysis in aqueous solutions using different temperatures (80, 100 and 120 °C) and observing the time dependence of the changes in the molecular weight. These authors observed that the hydrolytic degradation of ɣ-PGA was found to proceed through a random chain scission: at the highest temperature, faster and smaller molecules were obtained in a shorter time interval[3].Li et al. produced ɣ-PGA in large amounts (34.1 g/l) using Bacillus subtilis CGMC 2108 in a culture medium containing sodium glutamate at a concentration of 40 - 60 g/l[52].According to the existing literature, the ɣ-PGA was produced by Bacillus subtilis in batch fermentation using a medium with glycerol (20 g/l) as the carbon source supplemented with glutamic and citric acid, yielding 23 g/l after 30 h. This work was based on the fact that the addition of glycerol to the medium and the control of the pH have been shown to increase the carbon ɣ-PGA yield, findings that have not been studied in Bacillus subtilis. It was shown that, in a medium without glycerol supplementation with glutamic and citric acids, the maximum biomass and ɣ-PGA concentrations occurred at the initial pH values of 6.5 and 7.0 after 50 h of fermentation. When the initial pH was 6 or 8, the maximum biomass and ɣ-PGA production occurred after 90 h of fermentation in the same medium. Based on these results, a new study was conducted using glycerol in different concentrations, with the observation that the addition of glycerol had a positive effect on the maximum ɣ-PGA yield (23 g/l) after 30 h[37]. Bajaj and Singhal (2009) studied the effect of the addition of different amino acids and the acids of the tricarboxylic acid cycle as intermediate metabolic precursors for ɣ-PGA production in Bacillus licheniformis NCIM 2324. When the medium contained 0.5 mM L-glutamine and 10 mM α-ketoglutaric acid, the maximum yield was 35.75 g/l ɣ-PGA, whereas the maximum yield was 26.12 g/l ɣ-PGA in a medium without metabolic precursors. The medium culture was composed of glycerol, citric acid, L-glutamic acid, ammonium sulphate and other components. The initial pH was adjusted to 6.5, and the fermentation occurred for 96 h on a rotary shaker at 200 rpm and 37 ± 2 °C[1].Table 1 and 2 show the comparison between the different research conducted on the production of ɣ-PGA.
6. Conclusions
- This review showed that there are different methods of producing ɣ-PGA and that important consideration include the choice of microorganism and the different nutritional requirements and fermentation conditions. The large number of ɣ-PGA applications have been the impetus for many studies regarding its production. We also discussed the interesting alternative of the use of industrial residues to produce ɣ-PGA, a compound of interest to various industries and of environmental benefit.
ACKNOWLEDGEMENTS
- We would like to thank the CNPq for the PhD scholarship and for funding this project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
