-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2013; 3(1): 12-20
doi:10.5923/j.fph.20130301.02
Extraction of Polyphenols and Anthocyanins from the Jambul (Syzygium cumini) Fruit Peels
Diego T. Santos 1, Rodrigo N. Cavalcanti 1, Maurício A. Rostagno 1, Carmen L. Queiroga 2, Marcos N. Eberlin 3, M. Angela A. Meireles 1
1LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP, University of Campinas, 13083-862, Campinas, São Paulo, Brazil
2CPQBA/UNICAMP, Universityof Campinas, 13083-970, Campinas, São Paulo, Brazil
3Thomson Mass Spectrometry Laboratory, University of Campinas, 13083-970, Campinas, São Paulo, Brazil
Correspondence to: M. Angela A. Meireles , LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP, University of Campinas, 13083-862, Campinas, São Paulo, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work, the feasibility of using ultrasound alone and in combination with other techniques for the extraction of polyphenols and anthocyanins from jambul (Syzygiumcumini) peels was evaluated. The results were compared with conventional techniques (agitated bed and Soxhlet extraction techniques) based on total extract yields, total phenolic and monomeric anthocyanin concentrations in the extracts and the relative yields of these compounds. Ultrasound-assisted extraction was more efficient and selective for the extraction of anthocyanins than both conventional methods. When a combination of ultrasound and agitated bed extraction was used, a significant increase in total yields, and both polyphenol and anthocyanin concentrations was achieved. Electrospray Ionization Tandem Mass Spectrometry analysis indicated that diglycosideanthocyanins (cyanidin-3,5-diglycoside, peonidin-3,5-diglycoside, delphinidin-3,5-diglycoside, petunidin-3,5-diglycoside and malvidin-3,5-diglycoside) were the main polyphenols present in the samples.
Keywords: Polyphenols, Anthocyanins, Jambul, SyzygiumCumini, Ultrasound Assisted Extraction, Combined Extraction Techniques
Cite this paper: Diego T. Santos , Rodrigo N. Cavalcanti , Maurício A. Rostagno , Carmen L. Queiroga , Marcos N. Eberlin , M. Angela A. Meireles , Extraction of Polyphenols and Anthocyanins from the Jambul (Syzygium cumini) Fruit Peels, Food and Public Health, Vol. 3 No. 1, 2013, pp. 12-20. doi: 10.5923/j.fph.20130301.02.
Article Outline
1. Introduction
- In the last years, studies have increasingly associated the intake of fruits and vegetables with reduced risk for the development of chronic diseases such as cancer, diabetes type II, neurodegenerative disorders and cardiovascular diseases[1]. This phenomenon is likely influenced by biologically active molecules that are naturally present in those foods[2-4]. The interest in polyphenols is mainly due to their antioxidant properties; however, their mechanisms of action are known to be much more complex and to depend on several factors[5],[6]. Phenolic compounds are widely distributed in plants, although they are found in relatively high amounts in some plants, seeds and fruits, such as tea, yerba mate, cocoa, coffee, and grapes[7-10]. Many studies have been conducted in order to evaluate the biological activities of extracts of several usual and unusual sources of polyphenols. The major studies have investigatedantioxidant activity from extracts of tea[11],[12], curry[13] and fenugreek leaves[14], citrus peels[15] valerian (Valerianaofficinalis) root, lavender (Lavandulaofficinalis) and lemon balm (Melissa officinalis) leaves, (Alceakurdica) and (Stachyslavandulifolium) flowers[16], Aeglemarmelos leaves[17], wheat bran[18], among others. However, additional biological functions attributed to plant extracts have been analyzed such as antibacterial and antifungal[19], and anti-tumor[20],[21] activities. Polyphenols may also be present in high amounts in other less studied samples, such as other types of fruits available in certain countries. Among these fruits, jambul may represent a rich source of these compounds[22-25]. Jambul (Syzygiumcumini L.), also known as jambu, jamblon, jambula, jamboola, Java plum, jamun,jaam/kalojaam, jamblang, jambolan, blackplum, Damsonplum, Duhat plum, Jambolan plum, or Portuguese plum, belongs to the Myrtaceae family and has a small, egg-shaped purple fruit when ripe, with the pulp surrounding a single large seed. Several different phenolic classes have been reported in the fruits in high amounts[22-25]. Among these compounds, anthocyanins are one of the main polyphenols present, especially in the peels of the fruits. Anthocyanins are the largest and most important group of water-soluble and vacuolar pigments in nature. They are glycosylated polyhydroxy and polymethoxy derivatives of 2-phenylbenzopyrylium cation (Figure 1), i.e., the flavyliumcation (anthocyanidin group)[26]. Anthocyanins are of special interest because their antioxidant activity and their potential use in the food industry as natural colorants and preserving agents[27],[28].
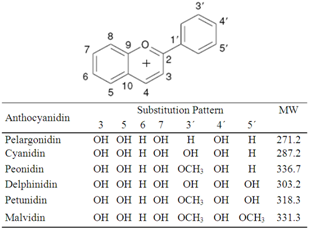 | Figure 1. Chemical structure of the main anthocyanidins found in nature |
2. Materials and Methods
2.1. Raw Material and Sample Preparation
- Jambul (Syzygiumcumini) fruits were obtained from the Institute of Biology at the University of Campinas (UNICAMP), Campinas, Brazil.The separation of peels, pulps and seeds was performed by crushing the whole fruit while avoiding damage to the seeds. Peels were washed with water to eliminate residual pulp. Samples were stored at -18℃ until submitted to the extraction procedures.
2.2. Chemical Reagents
- Ethanol (99.5%), methanol (99.5%), glacial acetic acid (99.7%) and sodium carbonate (99.5%) were obtained from Ecibra (Santo Amaro, Brazil). Ethyl acetate (99.5%) was purchased from Merck (Darmstadt, Germany). Hydrochloric acid (36.5-38%), sodium acetate (99%) and potassium chloride (99%) were obtained from Synth (Diadema, Brazil). Formic acid (98%) was purchased from Vetec (Rio de Janeiro, Brazil). Folin-Ciocalteu reagent was obtained from Dinâmica (Diadema, Brazil). Anthocyanin reference standards (cyanidin-3-glycoside chloride, delphinidin-3- glycoside chloride, peonidin-3-glycoside chloride, malvidin-3-glycoside chloride, pelargonidin-3- glycoside chloride and petunidin-3-glycoside chloride) and gallic acid were acquired from Extrasynthese (Genay, France). Reference standards of all analyzed compounds were HPLC grade with purities greater than 95%.
2.3. Extraction Procedures
- The samples were weighed and extracted using one of the different methods (Soxhlet, ultrasound, agitated bed and a combination of ultrasound and agitated bed). Soxhlet extractions were performed using 5 g of sample that was extracted with 120 mL of ethanol or acidified ethanol (acidified to pH 3 with HCl) (sample:solvent ratio of 1:25) for 8 hours at the boiling point of the solvent. The ultrasound-assisted extractions (UAE) were performed in an ultrasonic bath (40 kHz/81 W) (Thornton, T 1440, São Paulo, Brazil) using 2.5 g of the sample extracted with 25 mL of ethanol (sample:solvent ratio of 1:10) for 2 hours at room temperature. In a similar way, the agitated bed extractions (ABE) were performed in a rotary shaker (Marconi, MA 420, Piracicaba, Brazil) using 2.5 g of sample and 25 mL of ethanol (sample:solvent ratio of 1:10). Extractions were performed with agitation (150 rpm) for 2 hours at 30℃. Finally, a combination of ultrasound and agitated bed extraction (UAE + ABE) was used to improve the yields. Initially, 2.5 g of sample was extracted with 25 mL of ethanol (sample:solvent ratio of 1:10) during 10 minutes by UAE and submitted afterward to the same conditions of the ABE protocol. Independently of the method, the extracts were filtered, the solvent evaporated under vacuum (Laborota 4001, Heidolph Instruments GmbH, Viertrieb, Germany) at 40℃ and stored protected from light at -18℃ until its use in the chemical analysis.
2.4. Chemical Analyses
2.4.1. Total Phenolic Content(TPC)
- The content of total phenolic content(TPC) was determined using the Folin-Ciocalteau method, based on the colorimetric oxidation/reduction reaction of phenols[40]. Briefly, 1 mL of extract was mixed with 1 mL of Folin-Ciocalteu reagent. After 3 minutes, 1 mL of a saturated solution of sodium carbonate (50 %, w/w) was added to the mixture and the volume was adjusted to 10 mL with distilled water. The reaction mixture was kept protected from light for 90 minutes at room temperature, and afterwards, the absorbance was measured at 725 nm using a UV-Vis spectrophotometer (Hitachi, model U-3010, Tokyo, Japan). A blank was prepared using 1 mL of distilled water instead of the extract. The results were calculated based on the calibration curve of gallic acid (GA) (10.9-86.8 mg. L-1) and expressed as milligrams of Gallic Acid Equivalents (GAE) per gram of extract in dry base (d.b.) (mg GAE. g-1, d.b.). All determinations were made in triplicate.
2.4.2. Total Monomeric Anthocyanins (TMA)
- The total monomeric anthocyanin (TMA) content of the samples was determined using the pH differential method[41], which relies on the structural transformation of the anthocyanin chromophore as a function of pH. A UV-Vis spectrophotometer (Hitachi, model U-3010, Tokyo, Japan) and glass cuvettes of 1 cm in length were used to measure the maximum spectral absorption wavelength (512 nm and 700 nm) using distilled water as a blank. For this purpose, the sample was prepared with 20 mg of extract dissolved in 10 mL of distilled water. Two dilutions of this solution were prepared: one with hydrochloric acid/potassium chloride buffer (pH = 1.0) and the other with sodium acetate/acetic acid buffer (pH = 4.5). The pH values of the buffer solutions were measured using a pH meter (Digimed, model DM-22, Sao Paulo, Brazil) calibrated with buffers at pH 4.01 and 6.86, which were adjusted with HCl. Aliquots of the extract were measured and adjusted to pH 1.0 and 4.5 with the addition of buffer solutions. After 15 min, the absorbance of each equilibrated solution was measured at the maximum absorption wavelength and 700 nm for haze corrections using a 1 cm path length glass cell (l). The dilution factor (DF) was determined (the final volume divided by the original sample volume). The difference in absorbance values at pH 1.0 and 4.5 is directly proportional to the anthocyanin content. The total monomeric anthocyanin content (TMA) was calculated using cyanidin-3-glycoside as a reference (MW = 449.2 g mol-1 and ε = 26,900 L. mol-1.cm-1), and the results were expressed as mg cyanidin-3-glycoside equivalents/g of extract in dry base (d.b.) (mg cyn-3-gly equivalents g-1, d.b.). Relative standard deviations (RSDs) were lower than 8% for all samples. The absorbance of the diluted sample (A) and TMA were calculated with equations (1) and (2):
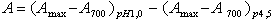 | (1) |
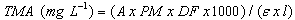 | (2) |
2.4.3. Thin-layer Chromatography (TLC)
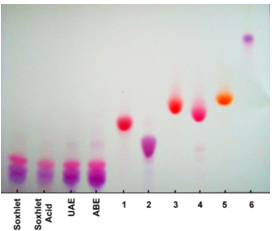
- For the determination of the qualitative chemical profile of the sample and its main polyphenols, the extract was fractionated using silica plates (20 × 20 cm, 1 mm height, Merck, Darmstadt, Germany). To identify the anthocyanins present in each extract, the mobile phase used was composed of ethyl acetate, glacial acetic acid, formic acid and distilled water (100:11:11:26), and no spray reagent was used[42]. Samples consisted of 10 mg of each extract dissolved in 1 mL of methanol. All anthocyanin standards were diluted in methanol to an approximate concentration of 5 mg. mL-1 and applied on the plates simultaneously with the sample.
2.4.4. Electrospray Ionization Tandem Mass Spectrometry (ESI-MS)
- A Q-TOF system (Micromass, Manchester, United Kingdom) was used for Electrospray Ionization Tandem Mass Spectrometry (ESI-MS/MS) analysis. The conditions used were a source temperature of 100℃, capillary voltage of 2.1 kV and cone voltage of 40 V. All data obtained from ESI in the positive ion mode were treated using Mass Lynx software (v.3.5, Waters, Manchester, United Kingdom). The samples (1 mg) were dissolved in methanol:water (1:1) with 0.7% formic acid; 1 µL of each sample was injected.
3. Results and Discussion
3.1. Polyphenols Extraction
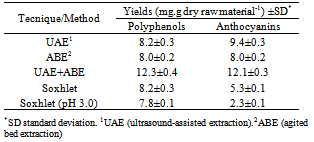
- Extraction of the sample was performed using different techniques, namely ultrasound-assisted extraction (UAE), agitated bed extraction (ABE), a combination of UAE and ABE (UAE+ABE) and Soxhlet extraction (using two different solvents). Extraction yields (%, d.b.) and polyphenol concentrations (mg of GAE/ g of extract, d.b.) are graphically illustrated in Figure 2.
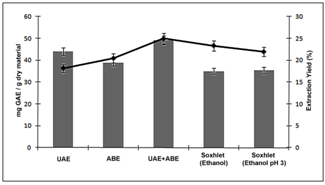 | Figure 2. Total phenolic content and extraction yield from extracts of jambul peels (Syzygiumcumini) obtained through various methods of extraction |
3.2. Anthocyanins Extraction
- Because anthocyanins have been reported to be the main polyphenols present in the peels of jambul[22],[24],[25], the influence of the extraction technique on the concentration of this compound class was also evaluated. Data for the concentrations of total monomeric anthocyanins (TMA) (mg of cyn-3-gly/ g of extract, d.b.) and the extraction yields (%, d.b.) obtained by different techniques are presented in Figure 3.
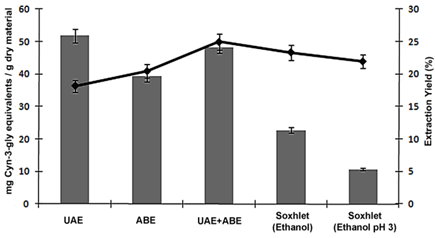 | Figure 3. Total monomeric anthocyanin content and extraction yield from extracts of jambul peels (Syzygiumcumini) obtained through various methods of extraction |
|
3.3. Anthocyanin Identification by Thin Layer Chromatography
- In addition to the comparison of the extraction techniques, it is important to individually identify the main compounds present in the sample to understand the extraction process and possible degradation patterns. Therefore, a qualitative analysis of the anthocyanins present was conducted initially by Thin Layer Chromatography (TLC) and later by Mass Spectrometry (MS).
3.4. AnthocyaninIdentification by Electrospray Ionization Tandem Mass Spectrometry (ESI-MS)
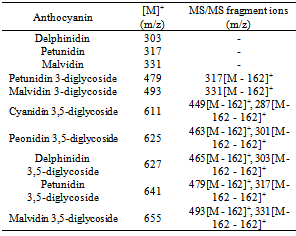
- ESI-Q-TOF-MSwas used to support the evidence provided by the TLC analysis of the samples. The mass spectrum obtained with analysis of the Soxhlet extraction sample (with acidified solvent) is presented in Figure 5. Extracts obtained by acidified solvent were only evaluated to establish a trusted anthocyanin identification. It is well known that the hydrolysis of the glycosides to their respective aglycone forms occurs under acidic conditions. The composition of anthocyanins from jambulanalysed by ESI-Q-TOF-MS are presented in Table 2.The identified compounds present in the extract were mainly anthocyanin diglycosides (cyanidin3,5-diglycoside (MW=611), peonidin3,5-diglycoside (MW=625), delphinidin3 ,5- diglycoside (MW=627), petunidin3,5- diglycoside (MW=641), malvidin3,5- diglycoside (MW=655)).These results are consistent with those reported in previous studies with jambul fruits[22-25]. However, it was detected anthocyaninsmonoglycosides (petunidin 3-diglycoside (MW = 479) and malvidin 3-diglycoside (MW = 493)). Furthermore,anthocyanidins (anthocyanin aglycone form) were also detected in the extracts obtained by Soxhlet extraction with acidified solvent (peonidin (MW=301), delphinidin (MW=303), petunidin (MW=317), malvidin (MW=331)). This result suggests that hydrolysis of the diglycosides to their respective aglycone forms may be taking place under these drastic conditions (acid/heat/long incubation time). On the other hand, the presence of mainly diglycosides supports the difference between TPC and TMA concentration due to the use of cyaniding 3-glycoside as a reference standard. Neither cyaniding 3-glycoside (MW=449) nor delphinidin3-glycoside (MW=465) was detected in the sample.
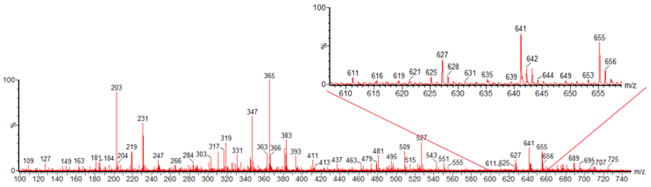 | Figure 5. Mass spectra data of sample from the Soxhlet with acidified solvent extractions |
|
4. Conclusions
- The data presented in this manuscript suggest that ultrasound is an attractive alternative to conventional methods for the extraction of polyphenols and anthocyanins from the peels of jambul. Extraction using conventional methods usually results in lower yields of polyphenols and anthocyanins from the sample and may lead to the degradation of these compounds during the extraction process. The results also provide additional information about the improvement of polyphenol extraction processes with the use of ultrasound as a combinatorial technique. Results suggest that ultrasound used in combination with other techniques can improve the selectivity and efficiency of the extraction of polyphenols and anthocyanins. Such information can be useful in the overall effort to reduce the time required to extract these compounds from samples.Thus, these informations set jambul as a new alternative source of bioactive compounds, especially due to high polyphenols and anthocyanins content and rich-anthocyanin profile implicating in its potencial commercial aplication as food colorant or to production of nutraceuticals and pharmacological products with benefits to human health.
ACKNOWLEDGEMENTS
- The authorsacknowledgethe financial supportfrom ConselhoNacional de DesenvolvimentoCientífico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Diego T. Santos would like to thank FAPESP (10/16485-5) for his postdoctoral fellowship.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML