-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(6): 301-308
doi: 10.5923/j.fph.20120206.16
“Flavor Intensity” Evaluation of Two Peach Fruit Accessions and their Four Offspring at Unripe and Ripe Stages by HS-SPME-GC/MS
Monica Bononi , Daniele Bassi , Fernando Tateo
Department of Agricultural and Environmental Sciences, Production, Landscape, Agroenergy, University of Milan, Milan, 20133, Italy
Correspondence to: Fernando Tateo , Department of Agricultural and Environmental Sciences, Production, Landscape, Agroenergy, University of Milan, Milan, 20133, Italy.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work successfully used an easy analytical method to produce comparable data referable to sensorial quality of fruits. In this study we examined the distribution of twenty-two volatile compounds for two peach accessions, Bolero and Oro A, and four of their offspring using head space solid-phase micro-extraction (HS-SPME) and GC-MS. The quantitative comparative distributions of thirteen “key volatile compounds” were elaborated to deduce the “flavour intensity” of each compound for each peach sample, also at two extreme stages (unripe and ripe). Clear differences were found between Oro A and Bolero and some trends have been evidenced considering their four offspring (for ex. total flavour intensities, hexyl-acetate trend, uniform decrease of (E)-2-hexenal). The criterion adopted permits to compare the flavours quality using “flavour intensity” data and to search for similar characteristics.
Keywords: Flavour Intensity, GC/MS, HS-SPME, Odor Threshold, Peach, Volatile Compounds
Cite this paper: Monica Bononi , Daniele Bassi , Fernando Tateo , "“Flavor Intensity” Evaluation of Two Peach Fruit Accessions and their Four Offspring at Unripe and Ripe Stages by HS-SPME-GC/MS", Food and Public Health, Vol. 2 No. 6, 2012, pp. 301-308. doi: 10.5923/j.fph.20120206.16.
Article Outline
1. Introduction
- The sensory quality of the peach (Prunus persica L. Batsch) is linked not only to basic organic components (sugars, organic acids, fibers, micro and macro elements) but also to flesh texture and in great part to the volatile compounds, which define the flavor impact. On the other hand, peaches are classified as either melting flesh (MF) or non-melting flesh (NMF) on the basis of fruit texture.In particular, the decrease in fruit firmness is more pronounced for MF fruits during ripening[1]. There are many studies concerning changes in the expression of genes in peaches [2-18], but there are few studies in the field on the identification of fundamental changes in volatile compound patterns during ripening for defined accessions or their offspring. Peach volatile compound patterns have been studied with different aims, including qualitative and quantitative distribution of volatiles[19, 20], characterization of peach and nectarine accessions from different origins[21, 22], changes in aroma composition during ripening and under different postharvest conditions[20, 23, 24], and interaction of volatiles with human receptors[25]. This work contributes to the study of fundamental modifications of volatile organic compounds and may represent an interesting model for studies on ripening, considering the fruit epicarp seems to be the tissue where most of the volatile compounds are produced[26].Different methods are used to extract the volatile fraction for aroma descriptions. The ones based on distillation, distillation/concentration, or on more complex capturing volatile systems seem to affect the extraction yield[21, 26-30] and don’t show very high advantages in comparison with more simple methods based on head space solid-phase micro-extraction (HS-SPME)[31-46]. In recent years, methods based on SPME are largely considered to be useful for comparative descriptions of volatile patterns, and able to offer a more representative image of the flavor impact on nose. For studies on production-transformation chains of fruits and derivatives, the use of simple analytical procedures for characterization of volatile profiles and rapid analysis systems may improve the standardization of quality and facilitate measures at various ripening stages. The SPME procedure involves the adsorption of analytes onto a fused silica fiber coated with suitable stationary phase and their subsequent desorption immediately before chromatographic analysis. The use of HS-SPME, i.e. exposing the fiber to sample head space, can drastically reduce interferences that affect the absorbance of flavor compounds on the fiber; consequently, the HS-SPME- GC/MS method permits a useful comparison of aroma profiles[33, 46-55]. In terms of fruit quality at consumption, harvest timing is the determining factor for consumer appreciation[23] and it is assumed that peach fruit expresses the best aroma when completely matured[56]. Consequently, the identification and quantification of volatile compounds are often considered only from ripe fruits[28]and many Authors, adopting various analytical approaches, demonstrate the general interest for the characterization of volatile compounds[21,26,28, 57-67]For the volatile compounds profile evaluation of two peach accessions known as Bolero (MF) and Oro A (NMF) and some of their offspring, the SPME technique was adopted. The two peach fruits have different phenotypes and were earlier objects of research on ethylene evolution[7]: the first is classified as melting flesh, ideal for fresh markets and the second is non-melting flesh, which is preferred for canning. The same two accessions have been studied for changes in the expression of genes involved in ethylene signalling[1, 8, 10]. In this study, is presented for the first time an evaluation model for volatile compounds based on the selection of key odorants involved in the sensorial changes that occur in peach accessions and their offspring during the ripening process. “Odor threshold” of key compounds were introduced to produce data of “flavor intensity” more objective and more useful than the per cent composition calculated from all volatile compounds.
2. Materials and Methods
2.1. Plant Material
- Two peach accessions (Bolero and Oro A) and four of their offspring, identified as BO 96 014 098 F, BO 96 014 137 F, BO 96 014 060 NF, and BO 96 014 106 NF (coded as “A”, “B”, “C”, and “D”, respectively), were grown following the fundamentals of integrated farming at the Experimental Orchard of the University of Milan (Azienda Agricola Zabina, Castel San Pietro, Bologna, Italy).
2.2. Ripe Stages
- Fruits at two ripe stages, unripe (U) and ripe (R), based on visual color changes and manual firmness of the fruit, were harvested from different trees. Ten (unripe and ripe) of each accession were chosen and kept for five days at 5℃ they were submitted to the analysis following theHS-SPME sampling, as described below.
2.3. HS-SPME Sampling
- The edible parts of the fruits were manually prepared, without using steel knives. Peels and pulps were homogenized, weighed, and separated into aliquots. Each sample (20 g) was poured into a 40 mL headspace vial fitted with a Teflon-lined septum. The SPME fibers(2cm of 50/30 DVB/CAR/PDMS) and the holder were obtained from Supelco, Italy, and were first conditioned according to the manufacturer’s instructions. A fiber was inserted into the headspace of the vial after 10 min at 20-25℃, to achieve headspace equilibrium, and was then exposed to the headspace for 20 min at 20-25℃, with magnetic stirring. Afterwards, the fibers were retracted into the needle and introduced into the GC/MS injector (T = 230℃) for desorption and analysis of the volatiles.
2.4. GC/MS Analysis
- The capillary GC/MS analyses were carried out using a gas chromatograph (Shimadzu GC-QP2010 model, coupled to a Shimadzu MS – QP2010). An Equity-5 capillary silica column (30 m x 0.25 mm, 0.25
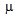 m film thickness) was used for compound separation. The column oven temperature was programmed as follows: 60℃ starting temperature (1 min), then 60–240℃ at 3℃/min heating rate, and 10 min hold. The carrier gas was He (1.0 mL/min), injection was in split mode (1/5), and the injector and detector temperatures were set to 230℃ and 250℃, respectively. The MS ran in electron impact (EI) mode at 70 eV electron energy, 1010 V electron multiplier, and 200℃ ion source temperature. Mass spectral data were acquired in scan mode (m/z range = 40–300). Identification was carried out by calculating retention indices and comparing mass spectra with those in databanks: in-house Di.S.A.A. library, NIST 147 (Shimadzu), and Adams[68].
m film thickness) was used for compound separation. The column oven temperature was programmed as follows: 60℃ starting temperature (1 min), then 60–240℃ at 3℃/min heating rate, and 10 min hold. The carrier gas was He (1.0 mL/min), injection was in split mode (1/5), and the injector and detector temperatures were set to 230℃ and 250℃, respectively. The MS ran in electron impact (EI) mode at 70 eV electron energy, 1010 V electron multiplier, and 200℃ ion source temperature. Mass spectral data were acquired in scan mode (m/z range = 40–300). Identification was carried out by calculating retention indices and comparing mass spectra with those in databanks: in-house Di.S.A.A. library, NIST 147 (Shimadzu), and Adams[68].3. Results and Discussions
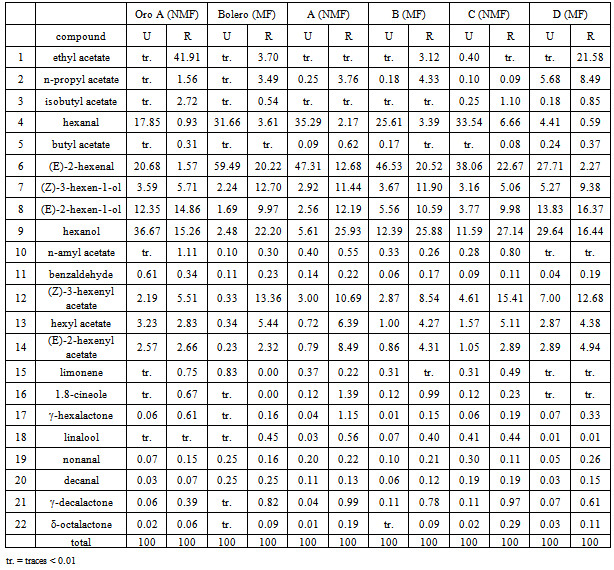
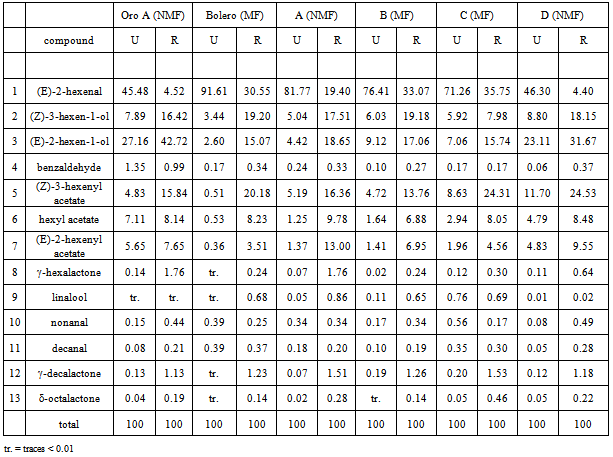
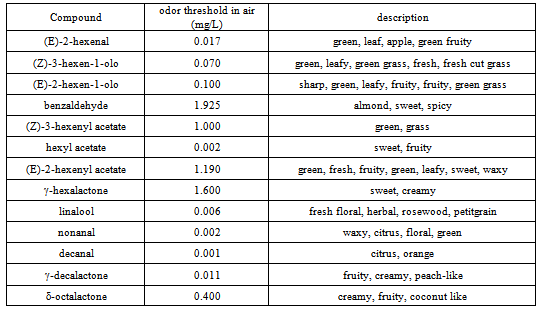
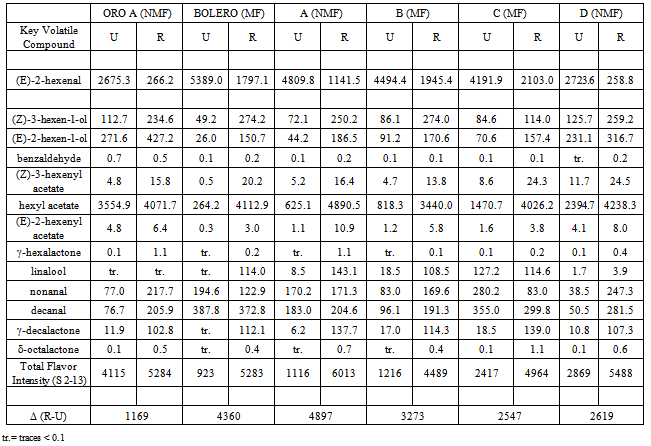
- The results of the GC/MS-SPME analysis were reported as area counts produced in identical conditions for the two peach accessions, Bolero (MF) and Oro A (NMF), and their four offspring. To guarantee the comparability of data, the adsorption efficiency of the fiber was controlled for each sample, verifying the repeatability (RSD < 3%)of the area of the standard substance (octanol-2). Table 1 shows the relative percentage composition of 22 volatile compounds on two extreme ripening stages (unripe and ripe, identified as “U” and “R”) for two accessionsOro A and Bolero and their four offspring. To obtain datarepresenting the aroma patterns with more correlation to sensorial perception, thirteen key volatile compounds were selected and new data were expressed as relative percentage of these selected compounds in Table 2. The list of these substances more specifically responsible of the peaches flavor appear in Table 3 with corresponding odor threshold (o.th) values and descriptive characters such as “green”, “grass”, “leaf”, “sweet”, “fruit”, “peach-like”.To obtain values named “flavorintensities”, the data shown in Table 2 were divided by the corresponding “odor threshold” (o.th.). The resulting data (%./o.th.)1-13, permit to write Table 4 where appears, for single column, the number of “odor unities” of thirteen single volatile compounds and the resulting “total flavor intensity”. Each column in fact represents numerically the odor unities present in an ideal volume (1 L ) of air containing 100mg of volatile compounds having the percentage composition reported in the corresponding column of Table 2. The amounts expressed as “total flavor intensities” do not include data concerning (E)-2-hexenal, identified as one of the two most quantitatively represented volatile compounds. The other one most represented compound is hexyl acetate.The two mentioned volatile compounds show an opposite trend: the first, shows in all cases decreasing values with the increase ripening, the second, on the contrary, shows increasing values from stage U to stage R. Therefore, the decreasing trend of (E)-2-hexenal with ripening corresponds to decrease of “green” note; the increasing trend of hexyl acetate produces sweet and fruity notes.Data concerning (E)-2-hexenal show to be comparable: Oro A with offspring D, while a lower likeness exists between Bolero, B and C.At stage “R”, comparable values of hexyl acetate correspond to Oro A, Bolero, A, C, D but with different values at “S” stage.The Δ(R-U) values represent a global measure of the quantitative modification of characteristic positive flavour notes occurring with ripening: an increase of this parameter is registered for all samples considered, but with some notable differences. The most evident increase is shown for Bolero, A and B, less evident for Oro A, C and D:One of the most characterizing notes (leafy, fruity, fresh cut gras and green grass) seems to be derived from the influence of alcohols (Z)-3-hexen-1-ol and (E)-3-hexen-1-ol. For this character, Oro A and Bolero appear to be very different, but the offspring A and B appear to be similar to Bolero. Considering in particular the trend of the sum of aldehydes nonanal and decanal it results to be evident a dissimilar comportment for different samples: with ripening it is evident the increase for samples Oro A, A, B, D but not for Bolero and C. Comparable data at stages “R” reach the sum of esters (including hexyl acetate).
|
|
|
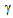 -decalactone, represent the most fruity, creamy, and peach-like volatile compounds and show a general increase from “U” to “R”, confirming the increase of fruity and creamy characteristic notes in correspondence to the ripe stage. The highest levels are reached by the lactones in samples A and C, but the levels corresponding to the other samples are not very lower.
-decalactone, represent the most fruity, creamy, and peach-like volatile compounds and show a general increase from “U” to “R”, confirming the increase of fruity and creamy characteristic notes in correspondence to the ripe stage. The highest levels are reached by the lactones in samples A and C, but the levels corresponding to the other samples are not very lower.4. Conclusions
- The results derived from the present study offer a useful opportunity to verify that HS-SPME technique coupled to the evaluation of the “flavor activity” allows a more realistic identification of flavors affecting sensorial characteristics during the ripening process of peach fruit. In effect, the data of per cent composition reported in Table 1 are not representative of flavor impact if not elaborated to obtain data of Table 4, which permits to attribute a more evident influence to some key compounds.The study of the possible likeness between accessions and their offspring, as far as the flavor impact is concerned, can be conducted evidently with more reference to reality if the solid phase extraction is adopted, which does not induce modifications of volatile compounds due to temperature or solvent effects. According to another Author[25], the study of volatile compounds is more correct and better corresponds to a sensorial quality evaluation if the techniques and results are focused on the identification of compounds with higher contributions to the aroma. As also affirmed by Grosch[25], less than 5% of the volatiles identified in foods contribute to aroma: therefore the Authors suggest to adopt the criterion of choice of key compounds and flavor intensity calculation to produce data better able to be employed for quality comparison effectively related to customer acceptance.When considering not just the absolute content of volatiles, but the ratio of analytical response to the odor threshold (i.e. flavor intensity values), the HS-SPME technique permits an easy characterization of the volatile compound composition with a better connection to the olfactory perception. The paper shows an example of important applications to compare two peach accessions and their offspring.
References
| [1] | E.A. Brovelli, J.K. Brecht, W.B. Sherman and C.A. Sims, “Potential maturity indices and developmental aspects of melting and non melting flesh peach genotypes for the fresh market”, Journal of the American Society for Horticultural Science, vol.123, no. 4, pp. 438-444, 1998. |
| [2] | T.G. Beckman, J.R. Alcazar, W.B. Sherman and D.J. Werner, “Evidence for qualitative suppression of red skin color in peach”, Hortscience, vol.40, no. 3, pp. 523-524, 2005. |
| [3] | C. Bonghi, L. Ferrarese, B. Ruperti, P. Tonutti and A. Ramina, “Endo-1,4-glucanases are involved in peach fruit grown and ripening, and regulated by ethylene”, PhysiologiaPlantarum, vol. 102, no. 3, pp. 346-352, 1998. |
| [4] | V. Dal Cin, F.M. Rizzini, A. Botton and P. Tonutti, “The ethylene biosynthetic and signal transduction pathways are differently affected by 1-MPC in apple and peach fruit”, Postharvest Biology and Technology, vol. 42, no. 2, pp. 125-133, 2006. |
| [5] | ESTree Consortium, “Development of an oligo-based microarray (PEACH1.0) for genomics studies in peach fruit”, ActaHorticulturae, vol. 682, pp. 263-268, 2005. |
| [6] | C. Etienne, A. Moing, E. Dirlewanger, P. Raymond, R. Monet and C. Rothan, “Isolation and characterization of six peach cDNAs encoding key proteins in organic acid metabolism and solute accumulation: involvement in regulating peach fruit acidity”, PhysiologiaPlantarum, vol. 114, no. 2, pp. 259-270, 2002. |
| [7] | A. Ghiani, N. Negrini, S. Morgutti, F.F. Nocito, A.M. Spinardi, C. Ortugno, I. Mignani, D. Bassi and M. Cocucci, “Flesh softening in melting flesh, non-melting flesh and stony hard peaches: endopolygalacturonase expression and phosphorylation of soluble polypeptides in relation to ethylene production”, in “Advances in Plant Ethylene Research: Proceedings of the 7th International Symposium on the Plant Hormone Ethylene”. A. Ramina et al. (Eds.), Springer Publishing, pp. 175-180, 2007. |
| [8] | A. Ghiani, F. Baldin, S. Morgutti, N. Negrini, F. F. Nocito, I. Mignani, D. Bassi and M. Cocucci, “Changes in the expression of genes involved in ethylene signalling in peach fruits with different flesh texture and softening patterns”, ActaHorticulturae (ISHS), vol. 884, pp.125-131, 2009. |
| [9] | F.M. Mathooko, Y. Tsunashima, W.Z.O. Owino, Y. Kubo and A. Inaba, “Regulation of genes encoding ethylene biosynthetic enzymes in peach (Prunuspersica L.) fruit by carbon dioxide and 1-methylcyclopropene”, Postharvest Biology and Technology, vol. 21, no. 3, pp 265-281, 2001. |
| [10] | S. Morgutti, N. Negrini, F.F. Nocito, A. Ghiani, D. Bassi and M. Cocucci, “Changes in endopolygalacturonase levels and characterization of a putative endo-PG gene during fruit softening in peach genotypes with nonmelting and melting flesh fruit phenotypes”, New Phytologist, vol. 171, no. 2, pp. 315-328, 2006. |
| [11] | B. Prinsi, A.S. Negri, C. Fedeli, S. Morgutti, N. Negrini, M. Cocucci and L. Espen, “Peach fruit ripening: a proteomic comparative analysis of the mesocarp of two cultivars with different flesh firmness at two ripening stages”, Phytochemistry, vol. 72, no. 10, pp. 1251-1262, 2011. |
| [12] | A. Rasori, B. Ruperti, C. Bonghi, P. Tonutti and A. Ramina, “Characterization of two putative ethylene receptor genes expressed during peach fruit development and abscission”, Journal of Experimental Botany, vol. 53, no. 379, pp. 2333-2339, 2002. |
| [13] | B. Ruperti, C. Bonghi, A. Rasori, A. Ramina and P. Tonutti, “Characterization and expression of two members of the peach 1-aminocyclopropane-1-carboxylate oxidase gene family”, PhysiologiaPlantarum, vol. 111, no. 3, pp. 336-344, 2001. |
| [14] | A. Tadiello, “A genomic investigation of the ripening regulation in peach fruit”. Ph.D. Thesis, University of Padova, Italy, 2010. |
| [15] | M. Tatsuki, T. Haji and M. Yamaguchi, “The involvement of 1-aminocyclopropane-1-carboxylic acid synthase isogene, Pp_ACSI, in peach fruit softening”, Journal of Experimental Botany, vol. 57, no. 6, pp. 1281-1289, 2006. |
| [16] | L. Trainotti, C. Bonghi, F. Ziliotto, D. Zanin, A. Rasori, G. Casadoro, A. Ramina and P. Tonutti, “The use of microarray PEACH1.0 to investigate trascriptome changes during transition from pre-climateric to climacteric phase in peach fruit”, Plant Sci. vol. 170, no. 3, pp. 606- 613, 2006. |
| [17] | L. Trainotti, D. Zanin and G. Casadoro, “A cell wall oriented genomic approach reveals a new and unexpected complexity of the softening in peaches”, Journal of Experimental Botany, vol. 54, no. 389, pp. 1821-1832, 2003. |
| [18] | V. Ziosi, M. Noferini, G. Fiori, A. Tadiello, L. Trainotti, G. Casadoro and G. Costa, “A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit”, Postharvest Biology and Technology, vol. 49, no. 3, pp. 319-329, 2008. |
| [19] | K. H. Engel, R.A. Flath, R.G. Buttery, T.R. Mon, D.W. Ramming, and R. Teranishi, “Investigation of volatile constituents in nectarines. 1. Analytical and sensory characterization of aroma components in some nectarine cultivars”, Journal of Agricultural and Food Chemistry, vol. 36, no. 3, pp. 549-553, 1988. |
| [20] | K.H. Engel, D.W. Ramming, R.A. Flath, R.G. and R. Teranishi. Investigation of volatile constituents in nectarines. 2. Changes in aroma composition during nectarine maturation, Journal of Agricultural and Food Chemistry, vol. 36, no. 5, pp. 1003-1006, 1988. |
| [21] | C. Derail, T. Hofmann and P. Schieberle, “Differences in key odorants of handmade juice of yellow-flesh peaches (Prunuspersica L.) induced by the workup procedure”, Journal of Agricultural and Food Chemistry, vol. 47, no. 11, pp. 4742-4745, 1999. |
| [22] | Y. Wang, C. Yang, S. Li, Y. Wang, J. Zhao and Q. Jiang, “Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS”, Food Chemistry, vol. 116, no. 1, pp. 356-364, 2009. |
| [23] | V. Farina, R. Lo Bianco and L. Di Marco, “Fruit quality and flavour compounds before and after commercial harvest of the late-ripening “Fairtime” peach cultivar”, International Journal of Fruit Science, vol. 7, no. 1, pp. 25-36, 2007. |
| [24] | A. Raffo, N. Nardo, M.R. Tabilio and F. Paoletti, “Effects of cold storage on aroma compounds of white and yellow-fleshed peaches”, European Food Research and Technology, vol. 226, no. 6, pp.1503-1512, 2008. |
| [25] | W. Grosch, “Evaluation of the key odorants of foods by dilution experiments, aroma models and omission”, Chemical Senses, vol. 26, pp. 533-545, 2001. |
| [26] | C. Aubert and C Milhet, “Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunuspersica L. Batsch)”, Food Chemistry, vol. 102, no. 1, pp. 375-384, 2007. |
| [27] | L.F. Di Cesare, R. Nani, N. Mariani and V. D’Angelo, “Influenza delle maltodestrine, ciclodestrine e dell’olio di palma sulla ritenzione degli aromi nei distillati di albicocca”, Industrie delle Bevande, vol. 25, pp.101-107. 1996. |
| [28] | I. Eduardo, G. Chietera, D. Bassi, L. Rossini and A. Vecchietti, “Identification of key odor volatile compounds in the essential oil of nine peach accessions”, Journal of the Science of Food and Agriculture, vol. 90, no. 7, pp. 1146- 1154, 2010. |
| [29] | E. Gomez and C.A. Ledbetter, “Development of volatile compounds during fruit maturation: characterization of apricot and plum x apricot hybrids”, Journal of the Science of Food and Agriculture, vol. 74, no. 4, pp. 541-546, 1997. |
| [30] | A. Polesello, L.F. Di Cesare and R. Nani, “Recupero degli aromi dagli ortofrutticoli mediante estrazione in fase solida”, Industrie delle Bevande vol.18, 93-101, 1989. |
| [31] | C. Arthur and J. Pawliszyn, “Solid phase microextraction with thermal desorption using fused silica optical fibers”, Analytical Chemistry, vol. 62, no. 19, pp. 2145-2148, 1990. |
| [32] | L. Ferreira, R. Parestrelo and J.S. Camara, “Comparative analisysis of the volatile fraction from Annonacherimola Mill. cultivars by solid-phase microextraction andchromatography-quadrupole mass spectrometry detection, Talanta, vol 77, no. 3, pp. 1087-1096, 2009. |
| [33] | D. Fraternale, D. Ricci, G. Flamini and G. Giomaro, “Volatile profiles of red apple from Marche Region (Italy)”, Records of Natural Products, vol.5, no. 3, pp. 202-270, 2011. |
| [34] | E. Ibañez, S. López-Sebastián, E. Ramos, J. Tabera and G. Reglero, “Analysis of volatile fruit components by headspace solid-phase microextraction”, Food Chemistry, vol. 63, no. 2, pp. 281-286, 1998. |
| [35] | M. Jia, Q. H. Zang and D.B. Min, “Optimization of solid-phase microextraction analysis for headspace flavor compounds of orange juice”, Journal of Agricultural and Food Chemistry, vol. 46, no. 7, pp. 2744-2747, 1998. |
| [36] | D. Komes, T. Lovrik and K.K. Ganic, “Aroma of dehydrated pear products”, LWT- Food Science and Technology, vol. 40, no. 9, pp. 1578-1586, 2007. |
| [37] | A. J. Matich, D.D. Rowan and N.H. Banks, “Solid phase microextraction for quantitative headspace sampling of apple volatiles” Analytical Chemistry, vol. 68, no. 32, pp. 4114- 4118, 1996. |
| [38] | J. Pawliszyn, “Theory of solid-phase microextraction”, Journal of Chromatographic Science, vol. 38, no. 7, pp. 270-278, 2000. |
| [39] | S.F.A.R. Reis, S.M. Rocha, A.S. Barros, I. Delgadillo and M. Coimbra, “Establishment of the volatile profile of “Bravo de Esmolfe” apple variety and identification of varietal markers”, Food Chemistry, vol. 113, no. 2, pp. 513-521, 2009. |
| [40] | M. Riu-Aumatell, M. Castellari, E. Lopez-Tamames, S. Galassi and S. Buxaderas, “Characterization of volatile compounds of fruit juices and nectars by HS/SPME and GC/MS” Food Chemistry, vol. 87, no. 4, pp.627-637, 2004. |
| [41] | E. Roth, A.Z. Berna, K. Beullens, S. Yarramraju, J. Lammertyn, A. Schenk and B.M. Nicolai, “Postharvest quality of integrated and organically produced apple fruit”, Postharvest Biology and Technology, vol. 45, no. 1, pp. 11-19, 2007. |
| [42] | M. Servili, R. Selvaggini, A. Tadicchi, A.L. Begliomini and G. Montedoro, “Relationship between the volatile compound evaluated by solid phase microextraction and the thermal treatment of tomato juice: optimization of the blanching parameters”, Food Chemistry, vol. 71, no. 3, pp. 407-415, 2000. |
| [43] | J. Song, B.D. Gardner, J.F. Holland and R.M. Beaudry, “Rapid analysis of volatile flavor compounds in apple fruit using SPME and GC/time-of-flight mass spectrometry”, Journal of Agricultural and Food Chemistry, vol. 45, no. 5, pp. 1801-1807, 1997. |
| [44] | J. Song, L. Fan and R.M. Beaudry, “Application of solid phase microextraction and gas chromatography/time-of-flight mass spectrometry for rapid analysis of flavor volatiles in tomato and strawberry fruits”, Journal of Agricultural and Food Chemistry, vol. 46, no. 9, pp. 3721-3726, 1998. |
| [45] | A. Steffen and J. Pawliszyn, “Analysis of flavor volatiles using headspace solid-phase microextraction”, Journal of Agricultural and Food Chemistry, vol 44, no. 8, pp. 2187- 2193, 1996. |
| [46] | F. Tateo and M. Bononi, “Headspace-SPME analysis of volatiles from quince whole fruits”, Journal of Essential Oil Research, vol. 22, no. 5, pp. 416-418, 2010. |
| [47] | P. Agozzino, G. Avellone, F. Filizzola, V. Farina and lo Bianco R, “Changes in quality parameters and volatile aroma compounds in “Fairtime” peach during fruit development and ripening”, Italian Journal of Food Science, vol. 19, no. 1, pp. 3-14, 2007. |
| [48] | F. Fernandes, P. Guedes de Pinho, P. Valentão, A.J. Pereira and P.B. Andrade, “Volatile constituents throughout Brassica oleracea L. var. acephala germination”, Journal of Agricultural and Food Chemistry, vol. 57, no. 15, pp. 6795-6802, 2009. |
| [49] | P. Guedes de Pinho, R.F. Gonçalves, P. Valentão, D.M. Pereira, R.M. Seabra, P.B. Andrade and M. Sottomayor, “Volatile composition of Catharanthusroseus (L.) G. Don using solid-phase microextraction and gas chromatography /mass spectrometry”, Journal of Pharmaceutical and Biomedical Analysis, vol 49, no. 3, pp. 674-685, 2009. |
| [50] | M. Meret, P. Brat, C. Mertz, M. Lebrun and Z. Günata, “Contribution to aroma potential of Andean blackberry (RubusglaucusBenth.)”, Food Research International, vol. 44, no. 1, pp. 54-60, 2011. |
| [51] | A.P. Oliveira, L.R. Silva, P. Guedes de Pinho, A. Gil-Izquierdo, P. Valentão, B.M. Silva, J.A. Pereira and P. B. Andrade, “Volatile profiling of Ficuscarica varieties by HS-SPME and GC-IT-MS”, Food Chemistry, vol. 123, no. 2, pp. 548-557, 2010. |
| [52] | U. Ravid, M. Elkabetz, C. Zamir, K. Cohen, O. Larkov and R. Aly, “Authenticity assessment of natural fruit flavour compounds in foods and beverages by auto-HS-SPME stereoselective GC-MS”, Flavour and Fragrance Journal, vol. 25, no. 1, pp. 20-27, 2010. |
| [53] | M. Taveira, P. Guedes de Pinho, R.F. Gonçalves, P.B. Andreade and P. Valentão, “Determination of eighty-one volatile organic compounds in dietary Rumexinduratus leaves by GC/IT-MS, using different extractive techniques”, Microchemical Journal, vol. 93, no. 1, pp. 67-72, 2009. |
| [54] | S. Vermeir, M.L.A.T.M. Hertog, K. Vankerschaver, R. Swennen, B.M. Nicolai and J. Lammertyn, “Instrumental based flavor characterization of banana fruit” LWT- Food Science and Technology, vol. 42, no. 10, pp.1647-1653, 2009. |
| [55] | S. Vichi, M. Riu-Aumatell, M. Mora-Pons, J. M. Guadayol, S. Buxaderas, E. López-Tamames, “HS-SPME coupled to GC/MS for quality control of Juniperuscommunis L. berries used for gin aromatization”, Food Chemistry, vol. 105, no. 4, pp.1748-1754, 2007. |
| [56] | C. Visai and M. Vanoli, “Volatile compound production during growth and ripening of peaches and nectarines”, ScientiaHorticolturae, vol. 70, no. 1, pp. 15-24, 1997. |
| [57] | C. Aubert, Z. Günata, C. Ambid and R. Baumes, “Changes in Physicochemical Characteristics and Volatile Constituents of Yellow- and White-Fleshed Nectarines during Maturation and Artificial Ripening”, Journal of Agricultural and Food Chemistry, vol. 51, no. 10, pp. 3083-3091, 2003. |
| [58] | C. Aubert, S. Baumann and H. Arguel, “Optimization of the analysis of flavor volatile compounds by liquid-liquid microextraction (LLME). Application to the aroma analysis of melons, peaches, grapes, strawberries, and tomatoes”, Journal of Agricultural and Food Chemistry, vol. 53, no. 23, pp. 8881-8895, 2005. |
| [59] | C. Aubert, and C. Chanforan, “Postharvest changes in physicochemical properties and volatile constituents of apricot (Prunusarmeniaca L.). Characterization of 28 cultivars”, Journal of Agricultural and Food Chemistry, vol. 55, no. 8, pp. 3074-3082, 2007. |
| [60] | F. Brandi, E. Bar, F. Morgues, G. Horvath, E. Turcsi, G. Giuliano, A. Liverani, S. Tarantini, E. Lewinsohn and C. Rosati, “Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism”, BMC Plant Biology, vol. 11, n. 24, pp. (26 January 2011), 2011. |
| [61] | M. Gonzáles-Aguero, S. Troncoso, o. Gudenschwager, R. Campos-Vargas, M.A. Moya-León and B.G. Defilippi, “Differential expression levels of aroma-related genes during ripening of apricot (Prunusarmeniaca L.)”, Plant Physiology and Biochemistry, vol. 47, no. 5, pp. 435-440,2009. |
| [62] | I. Jerković, Z., Marijanović and M.M. Staver, “Screening of Natural Organic Volatiles from Prunusmahaleb L. Honey: Coumarin and Vomifoliol as Nonspecific Biomarkers”, Molecules, vol. 16, no. 3, pp. 2507-2518, 2011. |
| [63] | J. Pereira, J. Pereira and J.S. Câmara, “Effectiveness of different solid-phase microextractionfibres for differentiation of selected Madeira island fruits based on their volatile metabolite profile - Identification of novel compounds”, Talanta, vol. 83, no. 3, pp. 899-906, 2011. |
| [64] | M. Staudt, B. Jackson, H. El-Aouni, B. Buatois, J.P. Lacroze, M.H. Sauge and U. Niinemets, “Volatile organic compound emissions induced by the aphid Myzuspersicae differ among resistant and susceptible peach cultivars and a wild relative”, Tree Physiology, vol. 30, no. 10, pp. 1320-1334, 2010. |
| [65] | Y. Wang, C. Yang, C. Liu, M. Xu, S. Li, L. Yang and Y. Wang, “Effects of bagging on volatiles and polyphenols in “Wanmi” peaches during endocarp hardening and final fruit rapid growth stages. Journal of Food Science, vol. 75, no. 9, pp. S455-S460, 2010. |
| [66] | W.-P. Xi, B. Zhang, L. Liang, J.-Y. Shen, W.-W. Wei, C.-J. Xu, A.C. Allan, I.B. Ferguson and K.-S. Chen, “Postharvest temperature influences volatile lactone production via regulation of acyl-CoA oxidases in peach fruit”, Plant, Cell and Environment, vol. 35, no. 3, pp. 534-545, 2012. |
| [67] | B. Zhang, J.-Y. Shen, W.-W. Wei, W.-P., Xi, C.-J. Xu, I.B. Ferguson and K.-S. Chen, “Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening”, Journal of Agricultural and Food Chemistry, vol. 58, no. 10, pp. 6157-6165, 2010. |
| [68] | R.P. Adams, “Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy”, Allured Publishing Corp. Carol Stream, IL, 1995. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML