-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(6): 276-280
doi: 10.5923/j.fph.20120206.13
Aspergillus Section Nigri in Grapes Cultivated in the Tropical Winery Region of Brazil
Fabiana Reinis Franca Passamani 1, Noelly Alves Lopes 2, Giuliano Elias Pereira 3, Guilherme Prado 4, Luis Roberto Batista 4
1Department of Food Science, Federal University of Lavras, Brazil
2Department of Biology, Federal University of Lavras, Brazil
3Embrapa Semi-arido, Petrolina/PE
4Ezequiel Dias Foundation, Belo Horizonte/MG, Brazil
Correspondence to: Fabiana Reinis Franca Passamani , Department of Food Science, Federal University of Lavras, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The main fungi responsible for the accumulation of ochratoxin A in grapes and wines belong to the genus Aspergillus Section Nigri. The production of this toxin by the fungus is related to abiotic factors and its presence may affect the quality and safety of the grapes grown and the wines produced. The aim of the present study was to assess the frequency of occurrence and the toxigenic potential of Aspergillus Section Nigri in grapes and seeds of Cabernet Sauvignon and Tempranillo varieties grown in the São Francisco Valley, northeast Brazil. To evaluate the distribution of these species and the toxigenic potential, samples of grapes of the Cabernet Sauvignon and Tempranillo varieties were collected in three wineries. The percentage of contamination in the grapes of the two varieties analyzed was larger than that of the seeds, and statistically different between the wineries studied. A total of 94 filamentous fungi were identified and none Aspergillus carbonarius were identified. The relationship between the percentage of contamination in the wineries examined and the geographic location and climate is not very clear, since the wineries are located on the same latitude and longitude, and are under influence of the same temperature (26℃).
Keywords: Aspergillus Section Nigri, Grapes, Seeds, Varieties, Temperature
Cite this paper: Fabiana Reinis Franca Passamani , Noelly Alves Lopes , Giuliano Elias Pereira , Guilherme Prado , Luis Roberto Batista , "Aspergillus Section Nigri in Grapes Cultivated in the Tropical Winery Region of Brazil", Food and Public Health, Vol. 2 No. 6, 2012, pp. 276-280. doi: 10.5923/j.fph.20120206.13.
Article Outline
1. Introduction
- Several species of filamentous fungi of the genus Aspergillus synthesize, as a secondary metabolite, a natural toxin called ochratoxin A. Ochratoxin A (OTA) is a highly stable chemical compound of low molecular weight with carcinogenic, immunosuppressive and teratogenic properties [1, 2] and is mainly associated with cereal processing[3, 4]. However, in recent decades, this toxin has also been observed in grapes and their derivatives[5, 6, 7, 8, 9], especially in regions of Europe, such as southern France, southern Italy and Greece[10].The production of this toxin by fungi is related to abiotic factors, especially temperature and water activity[11, 12, 13, 14] which can compromise the quality and safety of the wines.Agricultural products contaminated with mycotoxins, as well as causing risks to human and animal health, have a negative impact on the economy of many countries. Thus, knowledge about the conditions that govern the production of mycotoxins is extremely important for food quality control, since it can contribute to the establishment of preventive production measures, ensuring quality and safety of the final marketed product[15].The sub-medium region of the São Francisco Valley, northeast Brazil, began to operate in 1950 and was considered a new wine region when compared to the traditional wineries of the Old World and those already established in the United States, Australia, Chile and South Africa. The productivity of the vineyards is superior to the national and global averages, with ever-expanding crops and grapes of excellent quality, both for fresh consumption and for the production of wines, juices and other products[16].The area for growing wine grapes is estimated at 700 hectares of vines producing 6 million gallons of wine/year, of which 80% is red wine. The main varieties grown in this region for the production of fine red wines are Syrah, Tempranillo, Touriga, Petit Verdot and Cabernet Franc. These varieties are produced in characteristic climatic conditions, with a mean annual temperature of 26℃, relative humidity of 50%, average rainfall of 450 mm and an altitude of 365m[16]. Thus, this study aimed to evaluate the frequency of occurrence and the toxigenic potential of fungi of the genus Aspergillus Section Nigri in grapes and seeds of Cabernet Sauvignon and Tempranillo varieties grown in the São Francisco Valley, northeast Brazil, the only tropical wine region in the world.
2. Materials and Methods
2.1. Study Area
- The region has a tropical semi-arid climate with a mean temperature of 26℃, relative humidity of 50%, average rainfall of 450 mm and 300 sunny days/year. The area is larger than 360000 hectares, irrigated by the São Francisco River at an altitude of 365m[16].To assess the distribution of species of Aspergillus Section Nigri and their toxigenic potential, were collected, in 2010, samples of the grape varieties Cabernet Sauvignon and Tempranillo from three wineries located in the municipalities of Santa Maria da Boa Vista(09°04'42.8"S;40°08'19.7"W), Petrolina (09°02'10.7"S; 40°11'06.2"W) and Casa Nova (09°15'54.9"S; 40°51'11.0"W) in the sub-medium São Francisco Valley).
2.2. Isolation and Identification of Fungi
- In each winery we collected 5 bunches of grapes, at least 20m away from one another, along a 100m transect. These were conducted to the laboratory and placed in cold storage. Subsequently, 20 berries of each grape bunch were randomly selected and after surface disinfection, a total of 100 berries were plated directly on a culture medium Dichloran Rose Bengal Chloramphenicol (DRBC) and incubated at a temperature of 25℃ for a period of 7 days. This methodology was also used to seeds. After this period, the plates were analyzed to assess the percentage of contamination of grapes and seeds. Each plate was considered an independent sample. Only the colonies of interest were purified in Malt Extract Agar (MEA) at 25℃ for 5-7 days. All selected isolates were incubated in Czapek Yeast Agar (CYA) at standard temperatures of 25℃ and 37℃ and MEA at 25℃. After 7 days of incubation we observed the macro and microscopic characteristics of the filamentous fungi. From the pure cultures, fungi of the genus Aspergillus were identified using the methods by Klich and Varga et. al. [17, 18]. Purification and identification of filamentous fungi were performed in the Laboratory of Food Microbiology of the Departament of Food Science of the Federal University of Lavras, Brazil.
2.3. Determination of Ochratoxin a Production By Fungi Using the Agar Plug Method
- To determine the potential of ochratoxin A production, the isolates of the Section Nigri were inoculated in CYA at 25℃ for 7 days, after which they were submitted to the agar plug method as described by Filtenborg and Frisvad[19]. We used the OTA standard (Sigma-Aldrich), thin layerchromatography plates (Merck Silica Gel-60, 20x20) and as mobile phase, TEF – toluene ethyl acetate and formic acid 90% (60:30:10).
2.4. Analysis of Total Soluble Solids, Acidity and pH of the Grape Varieties
- For the chemical analysis of the grapes of each variety collected we performed an analysis of total soluble solids (TSS), measured as °Brix, pH and titratable acidity (TA), expressed as % tartaric acid. After obtaining the wine TSS analysis was performed with the aid of a portable digital refractometer. The acidity was obtained by titration with 0.1N NaOH using phenolphthalein indicator and the pH with a digital potentiometer.
2.5. Statistical Analysis
- To assess whether the grape varieties differed in the frequency of occurrence of Aspergillus section Nigri was employed an analysis of variance (ANOVA).
3. Results
3.1. Isolation and Identification of Fungi
- For the Cabernet Sauvignon variety, the mean percentage of contamination of grapes by Aspergillus section Nigri in Winery 1 was 82% and in Winery 2, 11%. In seeds, the mean values were lower when compared with the values obtained for the grapes. The mean contamination of seeds by Aspergillus section Nigri in Winery 1 was 57% and in Winery 2, only 1% (Figure 1). The analysis of variance showed that the difference in percentage of contamination by these fungi was statistically significant for both grapes (F = 75.14; p = 0.0002) and seeds (F = 247.58; p = 0.0005).
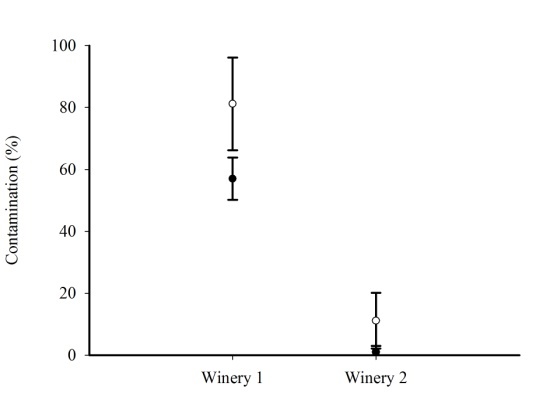 | Figure 1. Mean and standard deviation of grapes (○) and seeds (●) contaminated by Aspergillus Section Nigri of Cabernet Sauvignon in the two wineries |
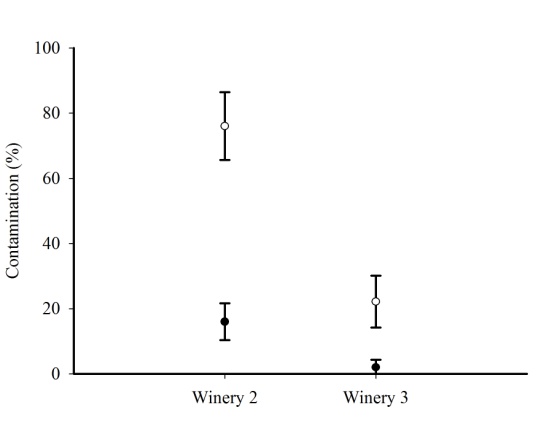 | Figure 2. Mean and standard deviation of grapes (○) and seeds (●) contaminated by Aspergillus Section Nigri of Tempranillo in the two wineries |
3.2. Analysis of Total Soluble Solids, Acidity and pH
- The data of the chemical composition of the grapes are presented in table 1. The values of titratable acidity (% of Tartaric acid) were significantly different between the Cabernet Sauvignon wineries (F = 36.80; p = 0.0261). However, the pH and total soluble solids were not statistically different. The Tempranillo variety in the two wineries showed the same values of titratable acidity and the values of pH and total soluble solids were not significantly different.
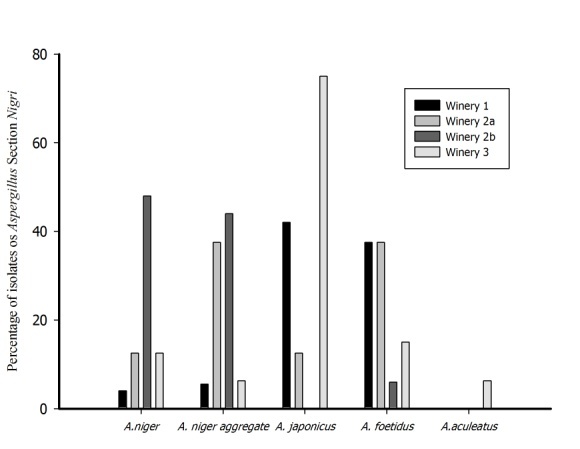 | Figure 3. Percentage of isolates of Aspergillus Section Nigri in the wineries |
4. Discussion
- The results of this study indicate that the grapes of the two varieties are more susceptible to contamination by Aspergillus section Nigri than the seeds, probably because the latter are protected inside the berry. In relation to the percentage of contamination between the wineries studied, even though[5, 20] attributed a high percentage of contamination in grapes grown in Portugal and Italy to the warm and dry climate, the role of geographical location and climate does not seem very clear in the current study, since the wineries sampled have the same latitude and longitude and are under influence of the same temperature (26℃).The higher percentage of tartaric acid found in the Cabernet Sauvignon variety grown in Winery 1 compared with the value obtained in Winery 2, may have favored the growth of filamentous fungi belonging to Aspergillus section Nigri. According to[20] grape varieties respond differently when artificially inoculated with Aspergillus carbonarius and, of the varieties studied, Cabernet Sauvignon was the most susceptible to these fungi. The content of tartaric acid in berries of the vine at harvest is related to temperature and, more specifically to the water content in the plant [21].The chemical composition of the Tempranillo variety had a low pH and acidity in grapes from both of the sampled wineries. According to[22] the grapes of Tempranillo variety grown in the sub-medium São Francisco Valley differed in their enological potential. The grapes harvested in November-December had high sugar content and pH and low acidity, while the grapes harvested in June-July had low sugar content and pH and high acidity. Thus, the characteristics of the grape varieties do not seem to explain these differences in contamination by Aspergillus section Nigri, as verified by[20] by inoculating fungi in different grape varieties in Italy. Thus, it seems that good agricultural practices such as application of fungicides, pest control and continuous irrigation, may be influencing these differences in contamination by fungi.Of the 94 fungi isolated and identified in the two varieties analyzed, 41 belong to the species Aspergillus niger and chromatographic analysis showed that no isolate produced ochratoxin A. Although species of the genus Aspergillus are cosmopolitan and grow in different types of climate and substrate no isolate of Aspergillus carbonarius was found in this study, demonstrating the low risk of contamination in wines made from grapes from this tropical region. Conversely, in temperate regions, Aspergillus carbonarius shows a high frequency of occurrence in ripe fruit, particularly grapes, as well as increased production of ochratoxin A[23].The location and climatic conditions of the wine region appear to influence the occurrence and frequency of ochratoxigenic species and, in countries located near the Mediterranean Sea and Australia, the species most commonly found in grapes are similar (Aspergillus niger and aggregate A. carbonarius)[7]. According to[24] 78.4% of the isolates of Aspergillus carbonarius evaluated had toxigenic potential in contrast to only 6.3% of the isolates of Aspergillus niger that produce ochratoxin A. On the other hand, Aspergillus niger is the main OTA-producing species in grapes produced in Argentina[25] although only a low percentage (5 to 10%) produce OTA[26].With regard to the species identified, studies of frequency of occurrence of fungi held in different grape producing regions noted that the following biseriate species, A. carbonarius, A. niger aggregate, A. niger, A. tubingensis and A. foetidus, and the following uniseriate species, A. aculeatus and A. japonicus, are predominant[5, 14, 27, 28]. The highest frequency of occurrence of these fungi in these regions can be explained by the color of their conidia that confer protection against sunlight and UV light, which would represent a competitive advantage for these species[17].
ACKNOWLEDGEMENTS
- The authors are grateful to CNPq and EMBRAPA semiárido for their financial support and for allowing the wineries to collect on their properties.
References
| [1] | IARC. 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans. Vol. 56, Lyon. |
| [2] | Murphy, P. A.; Hendrich, S.; Landgren, C, and Bryant, C. M. 2006. Food Mycotoxins: an update. Journal Food Science. 71, 51-65. |
| [3] | Vila, P. and Markaki, P. 2009. Aflatoxina B1 and ochatoxina A in breakfast cereals from Athens market: occurrence and risk assessment. Food Control, 20, 455-461. |
| [4] | Serratosa, M. P.; Lopes-Toledano, A.; Millan, C.; Medina, M. and Merida, J. 2010. Changes of Ochratoxin A in Grapes Inoculated with Aspergillus carbonarius and Subjected to Chamber-Drying under Controlled Conditions. Journal of Agricultural and Food Chemistry. 58, 11907-11912. |
| [5] | Serra, R.; Abrunhosa, L.; Kozakiewicz, Z. and Venâncio, A. 2003. Black Aspergillus species as ochratoxin A producers in Portuguese wine grapes. International Journal of Food Microbiology. 88: 63 – 68. |
| [6] | Battilani, P.; Giorni, P.; Bertuzzi, T.; Formenti, S. and Pietri, A. 2006. Black aspergilla and ochratoxin in grapes in Italy. International Journal of Food Microbiology. 111, 53-60. |
| [7] | Leong, S. L.; Hocking, A. D. and Scott E. C. 2006. Effect of temperature and water activity on growth and ochratoxin A production by Australian Aspergillus carbonarius and A. niger isolates on a simulated grape juice medium. International Journal of Food Microbiology. 110, 209-216. |
| [8] | Var, I. and Kabak, B. 2007. Ocurrence of ochratoxin A in Turkish wines. Microchemical Journal, 86, 241 – 247. |
| [9] | Valero, A.; Farre, J. R. and Sanchis, V. 2008. Survey: ochratoxin A in European special wines. Food Chemistry. 108, 593-599. |
| [10] | Magan, N.; Medina, A. e Aldred, D. 2011. Possible climate-change effects on mycotoxin contamination of food crops pre-and postharvest. Plant Pathology. 60, 150–163. |
| [11] | Quintela, S., Villarán, M. C.; Armentia, I. L. and Elejalde, E. 2011. Occurence of ochratoxin A in Rioja Alavesa Wines. Food Chemistry. 126: 302 – 305. |
| [12] | Selouane, A,; Bouya, D.; Lebrihi, A.; Decock, C. and Bouseta, A. 2009. Impact of Some Environmental Factors on Growth and Production of Ochratoxin A of/by Aspergillus tubingensis, A. niger, and A. carbonarius Isolated from Moroccan Grapes. The Journal of Microbiology. August, p. 411-419. |
| [13] | Pateraki, M.; Dekanea, A.; Mitchell, D.; Lydakis, D. and Magan, N. 2007. Influence on sulphur dioxide, controlled atmospheres and water availability on in vitro germination, growth and ochratoxin A production by strains of Aspergillus carbonarius isolated from grapes. Postharvest Biology and Technology. 44, 141-149. |
| [14] | Belli, N.; Ramos, A. J.; Sanchis, V. and Marin, S. 2004. Incubation time and water activity effects on ochratoxin A production by Aspergillus section Nigri strains isolated from grapes. Letters in Applied Microbiology. 38, 72-77. |
| [15] | Hussein, H. and Brasel, J. M. 2001. Toxicity, metabolism and impact of mycotoxin on human and animal. Toxicology. 167, 101-134. |
| [16] | Soares, J. M. and Leão, P. C. S. 2009. A Vitivinicultura no Semiárido Brasileiro. Embrapa Informação Tecnológica. Petrolina/PE. 756 pp. |
| [17] | Klich, M. A. 2002. Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures. |
| [18] | Varga, J,; Frisvad, J. C.; Koksubé, S.; Brankovics, B.; Tóth, B.; Sigeti, G. and Samsom, R. A. 2011. New and Revisited Species in Aspergillus Section Nigri. Studies in Mycology 69: 1- 17. |
| [19] | Filtenborg, O. and Frisvad, J. C. 1980. A simple screening method for toxigenic moulds in pure cultures. Lebensmittel Wissenschaft und Technologie, London, v. 13, p. 128-130. |
| [20] | Batillani, P.; Logrieco, A.; Giorni, P.; Cozzi, G.; Bertuzzi, T. and Pietri, A. 2004. Ochratoxin A production by Aspergillus carbonarius on some grape varieties grown in Italy. Journal of the Science of Food and Agriculture, 84:1736-1740. |
| [21] | Conde, C.; Silva, P.; Fontes, N.; Dias, A. C. P.: Tavares, R. M.; Sousa, M. J.; Agasse, A.; Delrot, S. and Gerós, H. 2007. Biochemical changes throughout grape Berry development and fruit and wine quality. Food. Japan, v. 1, n. 1, 1 – 22. |
| [22] | Pereira, G. E.; Guerra, C. C. and Alves, L. A. 2008. Avaliação da composição da uva e do vinho varietal ‘Tempranillo’ segundo a época de produção, na região do Vale do Submédio São Francisco. Anais do XII Congresso Brasileiro de Viticultura e Enologia, Bento Gonçalves, RS, Brasil. |
| [23] | Jorgensen, K. 2005. Occurrence of ochratoxin A in commodities and processed food – a review of EU occurrence data. Food Aditivies and Contaminants. v. 22, 26 – 30. |
| [24] | Tjamos, S. E.; Antoniou, P. P.; Kazantizidou, A.; Antonopoulus, D. F.; Papageorgiou, I. and Tjamos, E. C. 2004. Aspergillus niger and Aspergillus carbonarius in Corinth Raisin and Wine-Producing Vineyards in Greece: Population Composition, Ochratoxin A Production and Chemical Control. Journal of Phytopathology. 152, 250-255. |
| [25] | Perrone, G., Susca A., Cozzi, G., Ehrlich, K.; Varga, J.; Frisvad, J. C.; Meijer, M.; Noonim, P.; Mahakarrnchanakul, W. and Samson, R. A. 2007. Biodiversity of Aspergillus species in some important agricultural products. Studies in Mycology, 59: 53-66. |
| [26] | Perrone, G.; Gallo, A.; Susca, A. and Varga, J. 2008. Aspergillus in grapes: ecology, biodiversity and genomics. In: Aspergillus in the genomic era edited by Jãnos Varga and Robert Samsom. |
| [27] | Da Rocha Rosa, C. A.; Palacios, V.; Combina, M.; Fraga, M. E.; De Oliveira Reckson, A.; Magnoli, C. E. and Dalcero, A. M. 2002. Potential ochratoxin A producers from wine grapes in Argentina and Brazil. Food Aditivies and Contaminants. 19: 408 – 414. |
| [28] | Battilani, P., Pietri, A., Bertuzzi, T., Languasco, L., Giorni, P., Kozakiewicz, Z., 2003. Occurrence of ochratoxin A producing fungi in grapes grown in Italy. Journal of Food Protection. 66: 633-636. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML