-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(6): 231-240
doi: 10.5923/j.fph.20120206.08
Pressurized Organic Solvent Extraction with On-line Particle Formation by Supercritical Anti Solvent Processes
Diego T. Santos , Dayane F. Barbosa , Ketllen Broccolo , M. Thereza M. S. Gomes , Renata Vardanega , M. Angela A. Meireles
Lasefi/Dea/Fea (School of Food Engineering)/UNICAMP (University of Campinas) Cidade Universitária "Zeferino Vaz",R. Monteiro Lobato, 80; 13083-862, Campinas, SP, Brazil
Correspondence to: M. Angela A. Meireles , Lasefi/Dea/Fea (School of Food Engineering)/UNICAMP (University of Campinas) Cidade Universitária "Zeferino Vaz",R. Monteiro Lobato, 80; 13083-862, Campinas, SP, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work, anovel on-line process for pressurised hot organic solvent extraction of antioxidants from plantsas well as precipitation of the extract with or without a carrier material in one step was developed. This process has been called OEPO,Organic solvent Extraction and On-line particle formation. With this process, different products with a very low residual organic solvent concentration (< 50 ppm) can be obtained by the use of supercritical CO2 as anti solvent for solvent elimination.OEPO process consists of hyphenated Pressurized Liquid Extraction (PLE)-Supercritical Anti Solvent (SAS) precipitation, PLE-SAS co-precipitation and PLE-Supercritical Fluid Extraction of Emulsions (SFEE). OEPO process was successfully developed using Brazilian ginseng roots (Pfaffiaglomerata)as a model case using ethyl acetate as extracting solvent. Results were compared, in terms of antioxidant activity or morphology, with the ones obtained by each process separately.In addition, an optimization study for antioxidants recovery was performed using ethyl acetate as extracting solvent during PLE process. Optimum PLE extracts were produced under moderate extraction temperature (373 K) and high static extraction time (15 min). Under this condition an extraction yield of 1% (dry basis, d.b.) and an antioxidant activity of 53% are obtained, which was approximately 14% higherthan that observed after PLE-SAS precipitation and after SAS precipitation performed in two steps (step one - PLE extraction; step two – SAS precipitation by the use of the extract solution produced by step one stored).Similar behavior (hyphenated process producing similar products than the two step process done separately) was observed for PLE-SAS co-precipitation and PLE-SFEE indicating that the OEPO process developed in this work can be considered as a suitable and promising process to obtain, in only one step, different products (precipitated extract, co-precipitated extract or encapsulated extract in suspension), directly from plant materials.
Keywords: PLE, SAS, SFEE, Supercritical Fluids, Bioactive Compounds, Hyphenated Processes
Cite this paper: Diego T. Santos , Dayane F. Barbosa , Ketllen Broccolo , M. Thereza M. S. Gomes , Renata Vardanega , M. Angela A. Meireles , "Pressurized Organic Solvent Extraction with On-line Particle Formation by Supercritical Anti Solvent Processes", Food and Public Health, Vol. 2 No. 6, 2012, pp. 231-240. doi: 10.5923/j.fph.20120206.08.
Article Outline
1. Introduction
- Nowadays, the demand for natural bioactive compounds is increasing due to their use in the functional food industry. Natural components from plants are employed, including different functional activities, for instance,antioxidantactivity, antimicrobial activity, anti-cancer, or neurodegenerative diseases prevention, among others[1]. Ginseng species is one of the most appreciated natural sources for this kind of compounds.The most known Ginseng species in the world belongs to thePanax genus, which have been used for thousands years by folk medicine. Asian ginseng (Panax ginseng), American ginseng (Panax quinquefolius) roots arerenowned and widely used herbs in China, United States, Canada, etc.[2].Species of the genus Pfaffia (Amaranthaceae) has been commercialized as substitutes for Panax (ginseng, Araliaceae). Due to the similar morphology of its roots to those of ginseng, they are popularly known as “Brazilian ginseng”. Around 90 species of Pfaffia are known in Central and South America[3]. In Brazil, 27 species have been described, being Pfaffia glomerata the most important specie. Since besides similarity in appearance Brazilian ginseng roots (Pfaffia glomerata) extracts have also similar effects to ginseng, large amounts of this plant material are being exported for production of their extracts[4].Different classical extraction techniques have been applied to obtain antioxidant extracts from Pfaffia glomerata roots[5-7]. Classical extraction methods are time- and solvent-consuming and may promote extract degradation during the extraction process. On the other hand, pressurized liquid extraction (PLE) technique enables the rapid extraction (less than 30 min) of analytes in a closed and inert environment under high pressures (no higher than 20 MPa) and temperatures (298–473 K). A major advantage of PLE over conventional solvent extraction methods conducted at atmospheric pressure is that pressurized solvents remain in a liquid state well above their boiling points, allowing for high-temperature extraction. These conditions improve analyte solubility and the kinetics of desorption from matrices[8].The low stability during extraction, formulation, purification and storage of some class of bioactive compounds has influenced all these steps, which are being studied by different researchers interested in novel forms for processing these compounds with minimum degradation [9].The most important bioactive principles of Ginseng are saponins. Gradual degradation was observed with further increase in temperature resulting in complete destruction of saponins at temperatures > 543 K. Hydrothermolysis of triterpenoid and steroid saponins occurred upon heating in water at 473–513 K resulting in the production of aglycones, prosapogenins, and sugars[10,11]. Thus, the extracting solvent elimination step also should be done quickly and using mild operation conditions of temperature. Evaporation step usually expose the extracts to a risk of degradation of bioactive compounds catalysed by heat besides light and/or oxygen.In the search for alternative solvent elimination processes that can keep the stability of the extracted compounds, we have focused our attention on the use of supercritical fluids. Supercritical CO2 and organic solvents are miscible above a moderate pressure and temperature, while the compoundor class of compounds are not soluble in the mixture and it precipitates[12]. Denominated asSupercritical Anti Solvent (SAS) precipitation this method has been used extensively mainly to obtain small particles with narrow particle size distribution, which can be encapsulated, if required, by its co-precipitation together a carrier material (SASco-precipitation) or by the formulation of an emulsion [Supercritical Fluid Extraction of Emulsions (SFEE)][13,14].The encapsulation of natural substances presents several advantages over the natural substance itself. First, they acquire controlled release behaviour and are able to maintain their stability for longer periods[15].The development of hyphenated processes for combining bioactive compounds extraction with on-line particle formation is rarely reported. Ibanez group in Spain, recently, have developed a hyphenated process to obtain dried powders of extracts from natural sources in one step. This process called Water Extraction and Particle formation On-line (WEPO) similarly to ours also use PLE technique for bioactive compounds recovery, but employ water as extracting solvent. Since, in their case supercritical CO2 are not suitable for solvent elimination due to the low solubility of water in CO2, this fluid is used as a dispersion medium and hot N2 is used as drying agent[16]. Due to the similarities of WEPO process we have named our process asOrganic solvent Extraction and Particle formation On-line (OEPO). Differently of WEPO process, OEPO process also permits the encapsulation of the extract immediately after their production.Indeed, OEPO process consists of hyphenated PLE-SAS precipitation, PLE-SAS co-precipitation and PLE-SFEE.In this study the OEPO device and procedures are described in detail and successfully developed using Brazilian ginseng roots (Pfaffia glomerata) as a model case.Results were compared,in terms of antioxidant activity or morphology, with the ones obtained by each process separately. In addition, an optimization study for antioxidants recovery was performed using ethyl acetate as extracting solvent during PLE process and with static extraction time (5–15 min) and extraction temperature (353–413 K) as independent variables.The organic solvent ethyl acetate was chosen because it is a Generally Recognized as Safe (GRAS) solvent according to the US Food and Drug Administration (FDA) (toxicological class 3), it can be safely used in food applications[17,18].
2. Materials and Methods
2.1. Materials
- Brazilian ginseng roots (Pfaffiaglomerata) were cultivated in the experimental field of CPQBA (Campinas, Brazil), where they were collected on March 25, 2004, being 3 years old. They were washed and dried in a forced air circulation dryer at 313 K for 5 days. The dried roots (8.9% moisture) were then comminuted in a pulse mill (Marconi, model MA 340, Piracicaba, Brazil) for few seconds. Next, the particles of higher size were milled again, this time using a knife mill (Tecnal, model TE 631, Piracicaba, Brazil) for 2 s at 18,000rpm and finally, they were separated according to their size using sieves (Series Tyler, W.S. Tyler, Wheeling, IL). The milled roots were stored in freezer (Metalfrio, model DA 420, São Paulo, Brazil) at 263 K. For the extraction assays, particles of 7.89 µm of diameter, according to ASAE methodology[19], were used. The moisture content of the dried roots was determined by the AOAC method (Method 4.1.03)[20].Ethyl acetate (analytical grade) purchased from Merck (Darmstadt, Germany) was used as extracting solvent.Dichloromethane (analytical grade) purchased from Merck (Darmstadt, Germany) was used to prepare the Polyethylene glycol (PEG) solutions.Polyethylene glycol (PEG) with a mean molecular weight of 10,000 g/mol (mp: 336–338 K) (Sigma–Aldrich, Steinhein, Germany) was used as carrier material. N-octenyl succinic anhydride (OSA)-modified starch, kindly provided by National Starch Food Innovation (Hamburg, Germany), was used as surfactant and carrier material.Dry Carbon dioxide (CO2), 99.9% purity (Gama Gases Especiais Ltda., Campinas, Brazil)used as the antisolvent was supplied in the liquid phase.
2.2. Pressurized Liquid Extraction (PLE)
- The pressurized liquid extraction (PLE) system was designed and assembled at LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas). The solvent was pumped by a HPLC pump (Thermoseparation Products, Model ConstaMetric 3200 P/F, Fremoni, USA) into the extraction cell, which was placed in an electrical heating jacket at a desired temperature, until the required pressure was obtained.Dried and milled pieces of Brazilian ginseng roots (4.5 g with a moisture content of 8.9 %) were placed in a 6.57-cm3 extraction cell (Thar Designs, Pittsburg, USA) containing a sintered metal filter at the bottom and upper parts. The cell containing the sample was heated, filled with extraction solvent (ethyl acetate) and then pressurized. The sample was placed in the heating system for 6.5 min to ensure that the extraction cell would be at the desired temperature during the filling and pressurization procedure. After pressurization, the sample with pressurized solvent was kept statically at the desired pressure for the desired time (static extraction time). The pressure of the extraction was set in all experiments to 12 MPa to simulate the conditions of the OEPO process.Thereafter, the back pressure regulator (BPR) valve (Model #26–1761-24–161, Tesco, Elk River, USA) was carefully opened, keeping the pressure at an appropriate level for the desired flow (1.0 cm3/min), to rinse the extraction cell with fresh extracting solvent for 20 min (dynamic extraction time). After pressurized liquid extraction (PLE), the extracts were rapidly cooled to 268 K in ice water using glass flasks to prevent extract degradation. After extraction, depending on the aim different procedures were done. If the aim was to determine the extraction yield and the extract antioxidant activities, the solvent was evaporated using a rotary evaporator (Laborota, model 4001, Vertrieb, Germany) with vacuum control (Heidolph Instruments Gmbh, Vertrieb, Germany) and a thermostatic bath held at 313 K. On the other hand, if the aim was to use it in the particle formation processes, the 20 cm3 of ethyl acetate extract solution produced in each experiment were directly stored. All extracts (dried or not) were stored (263 K) in the dark prior to the next step (analysis or particle formation).
|
2.3. Supercritical Anti Solvent (SAS) Precipitation Process
- The experiments for ethyl acetate Pfaffiaglomerata roots extract precipitation were performed in a homemade Supercritical Anti Solvent (SAS) equipment designed and assembled at LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas)employing the ethyl acetate extract solution produced by PLE.Liquid CO2, antisolvent, was fed from the cylinder through athermostatic bath (MA-184, Marconi, Piracicaba, Brazil) at263 K to ensure the liquefaction of the gas and to preventcavitation, then it was pumped by an air-driven liquid pump(MaximatorGmbh, PP 111, Zorge, Germany) to the highpressurevessel (volume of 500 cm3; 6.8-cm internal diameter)via a nozzle.The nozzle consists of a 1/16” tube(inner diameter[i.d.]: 177.8millimeters) for the solution, placedinside a 1/8” tube for the CO2. Once the particle formationvessel reached steady state (temperature-313 K, pressure-10 MPa andCO2 flow rate-0.6 kg/h), the ethyl acetate extract solution was introduced into the vesselby a high-performance liquid chromatography (HPLC)pump (Model ConstaMetric 3200 P/F, ThermoseparationProducts, Fremont, USA) through the coaxial annular passageof the atomizer at a constant flow rate. The flow rate of the solution was set in all experiments to 1.0 cm3/min to simulate the conditions of the OEPO process. The vessel temperature (313 K) was maintained by a heating waterbath (MA 127BO, Marconi, Piracicaba, Brazil). CO2 flow rate (0.6 kg/h) was measuredusing a glass float rotameter (0.15–2.2 kg/h of CO2 at0.1013 MPa/293 K; 16/286A/2, ABB, Warminster, PA)coupled to a flow totalizer (Model G0,6, LAO, Osasco,Brazil). When the desired amount (20 cm3) of solution (ethyl acetate Pfaffiaglomerata roots extract) has been injected, which enabledthe collection of sufficient amount of precipitated powderfor analysis, the HPLC pump was stopped and only pureCO2 was fed. The flow of CO2 was maintained for 20 min forthe complete removal of the solvent from the precipitator,which was proven necessary by preliminary experiments.Pfaffia glomerata roots extract precipitates were trapped by apaper filter fixed at the bottom of the precipitation vessel while the fluidmixture (CO2+ethyl acetate) flowedto a second vessel (100-cm3 glass flask). At the end, the precipitation vessel was slowly depressurizedto atmospheric pressure and particles were collectedand stored in the dark in a domestic freezer (263 K;Double Action, Metalfrio, São Paulo, Brazil) until subsequentanalysis and characterization. A heating system maintained at 353 K was used to heat the micrometric valve toavoid the Joule–Thompson freezing effect that can lead toclogging of the throttling device during particle formationprocedure.
2.4. Supercritical Anti Solvent (SAS) Co-Precipitation Process
- The experiments for ethyl acetate Pfaffiaglomerata roots extract co-precipitation with PEG were done in the SAS equipment previously described. The SAS co-precipitation procedure is very similar tothe SAS precipitation, differing that a carrier material was added to theethyl acetate extract solution produced by PLE. PEG, in this case, was the carrier material and dichloromethane was the solvent.Dichloromethane was the selected solvent because it is a good solvent for PEG. Thus, in this case Pfaffia glomerata roots extract in PEG co-precipitates were trapped by a paper filter fixed at the bottom of the vessel while the fluid mixture (CO2+ethyl acetate+dichloromethane) exited the vessel.The operating condition were the same that during SAS precipitation (10 MPa and 313 K, CO2 flow rate of 0.6 kg/h, ethyl acetate solution flow rateof 1.0 cm3/min). The mass ratio between Pfaffiaglomerata roots extract and PEG investigated was 1:10
2.5. Supercritical Fluid Extraction of Emulsions (SFEE) Process
- Before initiating the SFEE process, an oil-in-water emulsion must be prepared. In general, these emulsions are prepared with the aid of surfactants.Twentycubic centimeterof the ethyl acetate solution produced by PLE was dispersed into 80 cm3 of an aqueous solution withOSA-modified starch surfactant (6 g/dm3) by the aid of a high speed-stirring mixer(IKA® magic LAB®, Staufen, Germany) with an engine power of 900 Watt processed during 4 min at 26.000 rpm. The mixer was cooled by ethylene glycol that circulates through a jacket, which allows to remove the heat generated by the equipment and to operate at temperatures lower than 298 K to avoid ethyl acetate evaporation.The SFEE experiments were done also in the SAS equipment previously described. The SFFE procedure and operating conditions were the same that during SAS precipitation and SAS co-precipitation, only differing that instead of a solution,an emulsion with ethyl acetate extract solution produced by PLEwas used as the dispersed phase. Then, in this case the suspension containing Pfaffia glomerata roots extract encapsulated in OSA-starch micelles remained in the precipitation vessel while the fluid mixture (CO2+ethyl acetate) exited.Afterwards, the suspensions obtained were further processed removing water to produce a dry powder. This was done by freeze-drying for 5 days at 60-100µHg and at -223K (Liobras, Liotop L101, São Carlos, Brazil).
2.6. Organic solvent Extraction and Particle Formation On-line (OEPO) Process
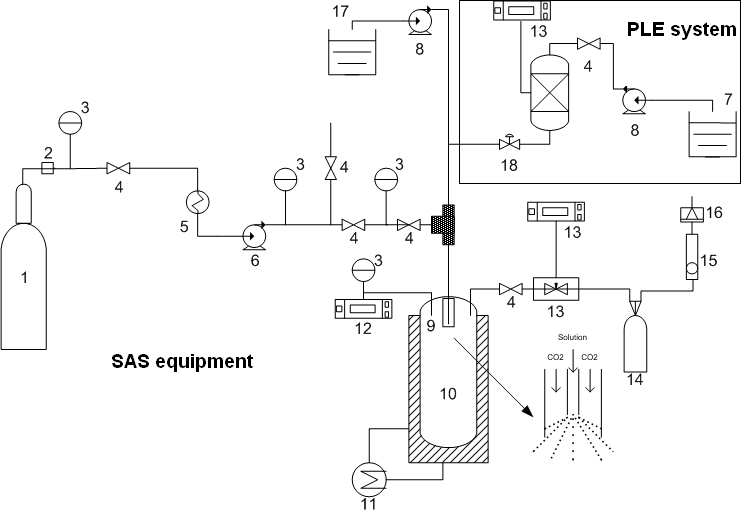
- The Organic solvent Extraction and Particle formation On-line (OEPO) process combines the two different processes previously described: firstly a dynamic PLE process using organic solvents and secondly the elimination of the solvent by the precipitation of the extract, using supercritical CO2 as a anti solvent. Thus, extraction and precipitation take place in the same system with a small time delay between these two processes.Figure 1 shows a scheme of the home-built equipment designed to carry out the organic solvent extraction with particle formation on-line (OEPO). From the extraction cell, the extract solution is led to a T- mixer where it can be mixed with a solution containing a carrier material dissolved also in an organic solvent or with an aqueous solution of surfactant. Shortly afterwards the solution or emulsion is exited through the coaxial annular passage of the atomizer together with supercritical CO2into the precipitation vessel.All connections used for coupling the PLE system with the SAS equipment were made using stainless steel tubes (1/16” and 1/8”).The extraction cell was filled with dried and milled pieces of Brazilian ginseng roots. The amount inserted of plant material was calculated in order to keep the same solvent volume to feed volume ratio employed during the previous PLE experiments (20 cm3 of solvent/4.5 g of roots). Theprocess started with a staticextraction period (selected after optimization) by filling the cellwith ethyl acetate at the desired temperature (selected after optimization)and pressure(12 MPa), with valve V18 closed (see Figure 1).At the same time, CO2 was pumped through the system at thedesired temperature (313 K) and pressure (10 MPa), with a constant flow rate(0.6 kg/h). The extraction continued in a continuous flow mode (dynamic extraction period) by opening valve V18 and setting the extracting solvent rate at the desired constant value. The ethyl acetate extract solution can meet first the solution containing PEG dissolved in dichloromethane or with an aqueous solution with OSA-modified starch surfactant (6 g/dm3) pumped with HPLC pump of the SAS equipment.The flow rate of both solutionswas set in order to achieve a constant total flow rate of ethyl acetate extract solution plus the resulted solution or emulsion of 1.0 cm3/min. When the aimed process was PLE-SAS precipitation, the second HPLC pump was turned off, since in this process there is no need of any carrier or surfactant material addition.Afterwards theorganic solvent (ethyl acetate) or solvents (ethyl acetate+dichloromethane) from the solution or emulsion areexited through the vessel precipitating the product, which can be a: i) precipitated extract (product after PLE-SAS precipitation); ii) co-precipitated extract(product after PLE-SAS co-precipitation) or iii)encapsulated extract in suspension (product after PLE-SFEE).The suspensions obtained by PLE-SFEE were further freeze-dried to produce a dry powder as previously described.
2.7. Product Characterization
2.7.1. Antioxidant Activity (AA)
- The evaluation of antioxidant activity of the extracts was based on the coupled oxidation of β-carotene and linoleic acid. The technique developed by Marco[21] consisted of measuring the bleaching of β-carotene resulting from oxidation by the degradation products of linoleic acid. In short, the substrate of reaction was prepared using 10 mg of β-carotene (97%, Sigma–Aldrich, St. Louis, USA), 10cm3 of chloroform (99%, Ecibra, Santo Amaro, Brazil), 60mg of linoleic acid (99%, Sigma–Aldrich, St. Louis, USA) and 200mg of Tween 40 (99%, Sigma–Aldrich, St. Louis, USA). This solution was concentratedin rotary evaporator (Laborota, model 4001, Vertrieb, Germany), with vacuum control (Heidolph Instruments Gmbh, Vertrieb, Germany) and a thermostatic bath at 323 K, being then diluted in 50cm3 ofdistilled water. The oxidation reaction was conducted using thefollowing procedure: to each 1 cm3 of substrate, 2 cm3 of distilled water and 0.05 cm3 of extract diluted in ethanol (99.5%, Ecibra, Santo Amaro, Brazil) were added. The dilution used for AA determination was 0.02 g of extract/cm3 of solvent. The mixture was placed in thermal bath (model TE 159, Tecnal, Piracicaba, Brazil Marconi, model MA159/300, Piracicaba, Brazil) at 313 K, and the product of reaction was monitored using a spectrophotometer (Femto, model 800 XI, São Paulo, Brazil Hitachi, model U-3010, Tokyo, Japan) at 0, 1, 2 and 3 h of reaction, using absorbance at 470 nm. The antioxidant activity was determined in duplicate for each extract and calculated following the same calculation procedure done by Santos et al.[22]. Antioxidant activity for synthetic BHT (at the same concentration that the extract) was also determined for comparison.
2.7.2. Microscopy
- Micrographs of the particles collected were taken by means of a scanning electron microscope (SEM) (LEO 440i, Leica, Cambridge, USA) after coating with a thin gold film with the aid of a sputter coater (Polaron, SC 7620, Ringmer, U.K.).
3. Results and Discussion
3.1. Effects of PLE Process Variables on the Extraction Yield and Antioxidant Activity
- The effects of extraction temperature and static extraction time on the extraction yield and on antioxidant activity were evaluated. The experimental values at various experimental conditions are presented in Table 2. In the variable ranges of 353–413 K and 5–15 min, the extraction yield variable wassignificantly (95% confidence level, p < 0.05) affected by extraction temperature, static extraction time and their interaction. On the other hand, only extraction temperature was significant (95% confidence level, p < 0.05) with respect to the antioxidant activity.The relationship of the extraction yield, extraction temperature and static extraction time was linear. An increase in either of temperature and static time, while the second variable remains constant, results in enhancement of the extract recovery. Moreover, the interaction between them had also a positive effect on the production of extract(Table 2). With regards of antioxidant activity, the increase of the extraction temperature beyond 373 K possibly might enhance the degradation of the bioactive compound extracted decreasing the antioxidant activity.
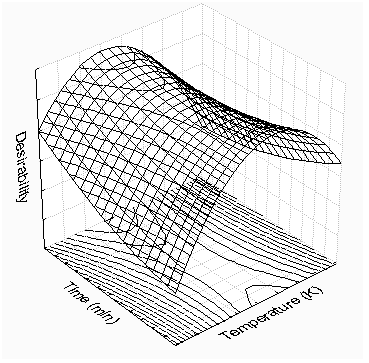 | Figure 2. Three-dimensional response surface of the influence of the PLEprocess variables on the desirability function |
3.2. Comparison betweenthe PLE Extracts, SAS Precipitated Extract and the Precipitated Extract Obtained from the OEPO Process
- The extract obtained under optimum PLE conditions was used as a control to compare antioxidant activity with the SAS precipitate extract and the precipitated extract obtained from the OEPO process (PLE-SAS precipitation)The pressure during the PLE processwere set in all experiments to 12 MPa, given that, this condition permits the coupling of SAS precipitation (and other anti solvent processes) after PLE.A slightly difference (14.24% lower) was observed between the PLE and OEPO samples, meaning thatthe OEPO process effects the antioxidantactivity of the extracts. SAS precipitation and OEPO process presented no significant difference (< 5%) between the samples, as expected. This difference between the samples can be attributed to the solubilization of some compounds in supercritical CO2 during anti solvent process[24]. Further improvements in our equipment in order to determine this loss will be done.A reference point for the anti solvent processes is the mixture critical point for the binary system CO2–organic solvent. In addition to the complete miscibility of the selected organic solvent in the supercritical anti solvent, a partial solubilization of some class of compounds can be aimed for fractionation purposes[25]. Recently, good results were obtained to fractionate the antioxidants from methanolic extract solution obtained from grape wastes without degradation and with the complete elimination of the solvent residues[26]. In our case, the fractionation phenomenon was undesired, but tuning the supercritical CO2density, we canprobably reduce the observed difference or even improve the antioxidant activity.
3.3. Comparison between the SAS Co-Precipitated Extract and the Co-Precipitated Extract Obtained from the OEPO Process
- Figure 3 shows the pictures of SAS co-precipitated extract and the co-precipitated extract obtained from OEPO process (PLE-SAS co-precipitation) obtained by scanning electron microscope (SEM).No significant differences were observed between the samples, meaning that the OEPO process does not have positive nor adverse effect on the morphology of the co-precipitated extracts.Recently, our research group evaluated the influence of several process variables during SAS co-precipitation of bixin-rich extract also in PEG[27]. Taking into account this study, we selected operating conditions that effectively encapsulate the extract minimizing extract and carrier material losses with supercritical CO2 flow. Obviously, as well as occurred during SAS precipitation the extract loss may have reduced the antioxidant activity. Once again, this antioxidant activity change can be avoided with the optimization of the anti solvent process.
3.4. Comparison between the Dried SFEE Encapsulated Extract and the Dried Encapsulated Extract Obtained from the OEPO Process
- OEPO process was also effective for the production of encapsulated extract. In Figure 4, SEM images of freeze-dried encapsulated extracts suspensions in water obtained by SFEE process and PLE-SFEE process, respectively. It is observed that both produced dried particles were spherical. Otherwise, the particles produced by OEPO process presented higher degree of porosity in their surface and higher particle diameter than SFEE particles.Briefly, in the SFEE process the emulsion and the supercritical CO2 are injected into the precipitation vessel continuously, and CO2 diffuses through the aqueous phase to the drop, extracting organic solvent out of the drop and precipitating the solute dissolved into the organic phase due to the anti solvent effect of the carbon dioxide[28].In SFEE process, like in the previously reported anti solvent processes, operating conditions, in general, are selected in order to facilitate maximum extraction of the organic phase with minimum extract and carrier material losses due to dissolution in CO2. In contrast, process variables like pressure and temperature are more closely related to the capacity to eliminate the remaining organic solvent from the products than to the final particle size[29]. The preparation of the emulsion has demonstrated to be the crucial point for the production of fine particles. The similarity between particle sizes of the initial emulsion and the final suspension suggests that the final particle size is dependent of the original droplet size of the emulsion[28-30]. Thus, the production of larger particles by OEPO process can be associated to the quality of the emulsion prepared using the T-mixer compared with that emulsion prepared using high speed-stirring mixer.
 | Figure 3. Scanning electron micrographs of: a) SAS co-precipitated extract; b) co-precipitated extract obtained from the OEPO process |
 | Figure 4. Scanning electron micrographs of: a) dried SFEE encapsulated extract; b) dried encapsulated extract obtained from the OEPO process |
4. Conclusions
- Organic solvent Extraction and Particle formation On-line (OEPO) process was described in detail and successfully developed using Brazilian ginseng roots (Pfaffia glomerata) as a model case. This novel process consists of an on-line process for pressurized hot organic solvent extraction of plant materials and precipitation of the extract with or without a carrier material by organic solvent elimination in one step, based on the use of supercritical CO2 as anti solvent.The use of PLE conditions employing ethyl acetate as extracting solvent for obtaining antioxidants from Pfaffia glomerata roots set at 12 MPa and 373 K under a static extraction time of 15 min was selected for further coupling with SAS precipitation, SAS co-precipitation with PEG and SFEE using OSA-modified starch as surfactant/carrier material. Indeed, OEPO process consists of hyphenated PLE-SAS precipitation, PLE-SAS co-precipitation and PLE-SFEE. Under this PLE condition an extraction yield of 0.934% (dry basis, d.b.) and an antioxidant activity of 52.96%were obtained, which was slightly higher (14.24%) that was observed after PLE-SAS precipitation and after SAS precipitation performed in two steps (step one - PLE extraction; step two –SAS precipitation by the use of the extract solution produced by step one stored). Similar behavior (hyphenated process producing similar products than the two step process done separately) was observed for PLE-SAS co-precipitation and PLE-SFEE indicating that the OEPO process developed in this work can be considered as a suitable and promising process to obtain, in only one step, different products (precipitated extract, co-precipitated extract or encapsulated extract in suspension) with desired antioxidant activity and particle size, directly from plant materials.
ACKNOWLEDGEMENTS
- Diego T. Santos would like to thank the FAPESP (2010/16485-5) for a postdoctoral fellowship. Maria Thereza M. S. Gomes and Renata Vardanega would like to thank CNPq for the doctoral and the master fellowships, respectively. Dayane F. Barbosa and Ketllen Broccolo are thankful to SAE/Unicamp. The authors acknowledge the financial support from CNPq(564721/2010-7) and FAPESP (2009/17234-9).
References
| [1] | M. Angela A. Meireles, Extracting Bioactive Compounds for Food Products: Theory and Applications, Contemporary Food Engineering series, CRC Press, 2008. |
| [2] | Guang-hua Lu, Qun Zhou, Su-qin Sun, Kelvin S. Leung, Hao Zhang, Zhong-zhen Zhao, "Differentiation of Asian ginseng, American ginseng and Notoginseng by Fourier transform infrared spectroscopy combined with two-dimensional correlation infrared spectroscopy", Elsevier,Journal of Molecular Structure, vol. 883-884, pp. 91-98, 2008. |
| [3] | Aline R. Zimmer, Fernanda Bruxel, Valquíria L. Bassani, Grace Gosmann, "HPLC method for the determination of ecdysterone in extractive solution from Pfaffiaglomerata", Elsevier, Journal of Pharmaceutical and Biomedical Analysis, vol. 40, pp. 450-453, 2006. |
| [4] | I. M. Júnior, "Avaliação de genótipos de Pfaffiaglomerata (Spreng.) Pedersenvisando o seucultivocomercial", Master's degree dissertation, Agronomic Institute of Campinas, Brazil, 2005. |
| [5] | Cristina S. Freitas, Cristiane H. Baggio, Samanta L. Araújo, Maria C. A. Marques, "Effects of Pfaffiaglomerata (Spreng) Pedersen Aqueous Extract on Healing Acetic Acid-induced Ulcers", Scielo, Brazilian Archives of Biology and Technology, vol. 51, no. 4 : pp. 679-683, 2008. |
| [6] | Cristina S. Freitas, Cristiane H. Baggioa, José E. Silva-Santos, LiaRiecka, Cid A. M. Santos, Cirino C. Júnior, Lin C. Ming, Diógenes A. G. Cortez, Maria C. A. Marques, "Involvement of nitric oxide in the gastroprotective effects of an aqueous extract of Pfaffiaglomerata (Spreng) Pedersen,Amaranthaceae, in rats", Elsevier, Life Sciences, vol. 74, pp. 1167-1179, 2004. |
| [7] | Rejane Flores, Fernando T. Nicoloso Daniela Brondani, JoseilaMaldaner, VercianeCezarotto, Sandro R. Giacomelli, "Extraction of ecdysterone from roots of Brazilian ginseng", Scielo, Ciência Rural, vol. 39, no. 4, pp. 1223-1226, 2009. |
| [8] | Diego T. Santos, Priscilla C. Veggi, M. Angela A. Meireles, "Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins", Elsevier, Journal of Food Engineering, vol. 108, pp. 444-452, 2012. |
| [9] | Diego T. Santos, Juliana Q. Albarelli, Marisa M. Beppu, M. Angela A. Meireles, "Stabilization of anthocyanin extract from jabuticaba skins by encapsulation using supercritical CO2 as solvent", Elsevier, Food Research International. in press. |
| [10] | OzlemGuclu-Ustundag, Giuseppe Mazza, "Extraction of saponins and cyclopeptides from cow cockle seed with pressurized low polarity water", Elsevier, LWT-Food Science and Technology, vol. 41, pp. 1600-1606, 2008. |
| [11] | OzlemGuclu-Ustundag, John Balsevich, Giuseppe Mazza, "Pressurized low polarity water extraction of saponins from cow cockle seed", Elsevier, Journal of Food Engineering, vol. 80, pp. 619-630, 2007. |
| [12] | Ernesto Reverchon, "Supercritical antisolvent precipitation of micro- and nano-particles", Elsevier, The Journal of Supercritical Fluids, vol.15, no.1, pp.1-21, 1999. |
| [13] | María J. Cocero, Ángel Martín, FacundoMattea, SalimaVarona,"Encapsulation and co-precipitation processes with supercritical fluids: fundamentals and applications", Elsevier,The Journal of Supercritical Fluids, vol.47, no.3, pp.546-555, 2009. |
| [14] | Diego T. Santos, Ángel Martín, M.Angela A. Meireles, María José Cocero, "Production of stabilized sub-micrometric particles of carotenoids using supercritical fluid extraction of emulsions", Elsevier, The Journal of Supercritical Fluids, vol.61, pp.167-174, 2012. |
| [15] | Diego T. Santos, M. Angela A. Meireles, "Carotenoid pigments encapsulation: fundamentals, techniques and recent trends", Bentham Open, The Open Chemical Engineering Journal", vol.4, pp.42–50, 2010. |
| [16] | Maria E. E. Ibanez, I., Alejandro Cifuentes., Irene Rodriguez-Meizoso, José A. Mendiola, GuillermReglero, Javier Señorans, Charlotta Turner, Device and process for the on-line extraction and drying of complex extracts, Spanish Patent no. P200900164, 2009. |
| [17] | Esther Paz, Ángel Martín, Antonio Estrella, Soraya Rodríguez-Rojo, Ana A. Matias, Catarina M. M. Duarte, María José Cocero, "Formulation of β-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant", Elsevier, Food Hydrocolloids, vol.26, no.1, pp.17–27, 2012. |
| [18] | Miguel A T, Cardoso, Sofia Antunes, Frederik V. Keulen, Bruno S Ferreira, Augusto Geraldes, Joaquim M. S. Cabral, António M. F. Palavra, "Supercriticalantisolventmicronisation of synthetic all-trans-β-carotene with tetrahydrofuran as solvent and carbon dioxide as antisolvent", Elsevier, Journal of Chemical Technology and Biotechnolology, vol. 84, pp. 215-222, 2009. |
| [19] | ASAE–American Society of Agricultural Engineers, "Method of determining and expressing fineness of feed materials by sieving" , in: American Society of Agricultural Engineers Standard, ASAE–American Society of Agricultural Engineers, pp. 447- 448, 1993. |
| [20] | AOAC-Association of Official Analytical Chemists, in: P. Cuniff (Ed.), AOAC International, Gaithersburg, Maryland, 1997. |
| [21] | Gino J. Marco, "A rapid method for evaluation of antioxidant", Springer, Journal of The American Oil Chemist’s Society vol. 45, pp. 594, 1968. |
| [22] | Diego T. Santos, Priscilla C. Veggi, M. Angela A. Meireles, "Extraction of antioxidant compounds from jabuticaba (Myrciariacauliflora) skins: yield, composition and economical evaluation", Elsevier, Journal of Food Engineering, vol. 101, pp. 23-31, 2010. |
| [23] | Patrícia F. Leal, Marina B. Kfouri, Fábio C. Alexandre, Fábio H. R. Fagundes, Juliana M. Prado, Marcos H. Toyama, M. Angela A. Meireles, "Brazilian Ginseng extraction via LPSE and SFE: Global yields, extraction kinetics, chemical composition and antioxidant activity", Elsevier, Journal of Supercritical Fluids, vol. 54, pp. 38-45, 2010. |
| [24] | Owen J. Catchpole, John.B. Grey, KeithA. Mitchell, Jiansheng S. Lan "Supercritical antisolvent fractionation of propolis tincture", Elsevier, Journal of Supercritical Fluids, vol. 29, pp. 97-106, 2004. |
| [25] | Yueh-Cheng Cho, Jia-Hui Cheng, Shih-Lan Hsu, Siang-En Hong, Tse-Min Lee, Chieh-Ming J. Chang, "Supercritical carbon dioxide anti-solvent precipitation of anti-oxidative zeaxanthin highly recovered by elution chromatography from Nannochloropsisoculata", Elsevier, Separation and Purification Technology, vol. 78, 274-280, 2011. |
| [26] | TomasFloris, GiorgiaFilippino, StefaniaScrugli, Maria B. Pinna, Francesca Argiolas, AntonioArgiolas, Mariano Murru, Ernesto. Reverchon, "Antioxidant compounds recovery from grape residues by a supercritical antisolvent assisted process", Elsevier, Journal of Supercritical Fluids, vol. 54, 165-170, 2010. |
| [27] | Diego T. Santos, M. Angela A. Meireles, "Micronization and encapsulation of functional pigments using supercritical carbon dioxide", Wiley, Journal of Food Process Engineering, in press. |
| [28] | PratibhashChattopadhyay, Boris Y. Shekunov, Jeff S. Seitzinger, Robert W. Huff, Particles from supercritical fluid extraction of emulsion, US Patent no0026319 A1, 2004. |
| [29] | FacundoMattea, Ángel Martín, Constantin Schulz, Philip Jaeger, Rudolf Eggers, María J. Cocero,"Behavior of an organic solvent drop during the supercritical extraction of emulsions", American Institute of Chemical Engineers, AIChE Journal, vol.56, no.5, pp.1184-1195, 2010. |
| [30] | FacundoMattea, Ángel Martín, AránMatías-Gago, María J. Cocero,"Supercritical antisolvent precipitation from an emulsion: β-Carotene nanoparticle formation", Elsevier, The Journal of Supercritical Fluids, vol.51, no.2, pp.238-247, 2009. |
| [31] | Benjamin Brocart, Philippe A. Tanguy, César Magnin, Jacques Bousquet, "Design of In-Line Emulsification Processes for Water-in-Oil Emulsions",Taylon&Francis,Journal of Dispersion Science and Technology, vol. 23, no. 1-3, 2002. |
| [32] | Fernando. Miguel, Ángel Martín, FacundoMattea, Maria J. Cocero,"Precipitation of lutein and co-precipitation of lutein and poly-lacticacid with the supercriticalanti-solvent process", Elsevier, Chemical Engineering and Processing: Process Intensification, vol, 47, no. 9-10, pp. 1594-1602, 2008. |
| [33] | Aaron S. Mayo, Balamurali K. Ambati, Uday B. Kompell, "Gene delivery nanoparticles fabricated by supercritical fluid extraction of emulsions", Elsevier, International Journal of Pharmaceutics, vol. 387, pp. 278-285, 2010. |
| [34] | Giovanna D. Porta, Ernesto Reverchon, "Nanostructured Microspheres Produced by Supercritical Fluid Extraction of Emulsions", Wiley, Biotechnology and Bioengineering, vol. 100, no. 5, pp. 1020-1033, 2008. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML