-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(5): 178-183
doi: 10.5923/j.fph.20120205.09
Amaranth Grain Brings Health Benefits to Young Normolipidemic Rats
Cinthia Baú Betim Cazarin 1, Yoon Kil Chang 2, Matheus Depieri 2, Everardo Magalhães Carneiro 3, Aparecida Sônia de Souza 4, Jaime Amaya-Farfan 1
1Department of Food and Nutrition, School of Food Engineering – University of Campinas, Campinas, 13083-862, Brazil
2Department of Food Technology, School of Food Engineering – University of Campinas, Campinas, 13083-862, Brazil
3Department of Anatomy, Cell and Molecular Biology, Institute of Biology – University of Campinas, Campinas, 13083-865, Brazil
4Institute of Food Technology – ITAL, Campinas, 13070-178, Brazil
Correspondence to: Cinthia Baú Betim Cazarin , Department of Food and Nutrition, School of Food Engineering – University of Campinas, Campinas, 13083-862, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This paper reports the impact of an extruded amaranth (Amaranthus spp) supplement on intestinal bile and fatty acids of normolipidemic rats. Forty-eight rats were fed either a control diet AIN 93-G (12 or 18% protein), or one of two levels of supplemental extruded amaranth flour for 48 days. Plasma glucose, insulin, total cholesterol, their fractions, and triacylglycerols, liver weight and lipid content, cecum short-chain fatty acids and fecal excretion of bile acids were determined. While no significant differences in the levels of plasma triacylglycerols, glucose or insulin, and liver parameters due to the diet were detected. Although amaranth promotes reduction of both total and LDL serum cholesterol, increased production of butyric acid in cecum and fecal excretion of deoxycholic acid in the feces. These results suggest that amaranth used routinely as a supplement to the standard casein diet could have a health-promoting value in the normal rat.
Keywords: Amaranthus cruentus, Dietary Fiber, Dyslipidemia, Cholesterol, Secondary Bile Acids, Short-Chain Fatty Acids
Article Outline
1. Introduction
- Health studies reveal that metabolic disorders continue to increase in human populations world-wide. In the search for healthier foods and life styles, less common foods have received special attention and, among them, amaranth grain has a number of unique nutritional features to offer.Amaranth grain is a pseudo-cereal crop known in the Americas for such agronomic characteristics as resistance to drought and abrupt temperature changes, as well as tolerance to salt and pH variations in the soil. For centuries it has been a staple food to those in Inca, Maya and Aztec civilizations, but in more recent times, its unusual compositional characteristics have promoted a number of researchers to further explore its health properties[1]. The grain's protein content may reach 15% and the balance between lysine and the sulfur amino acids approaches that of a mixture of rice and beans[2-3]. Its lipid fraction (6 to 10%) has a high content of unsaturated fatty acids (76%), principally linoleic acid and includes considerable amounts of squalene. Additionally, between 56 and 78% of the dry matter is starch, and from 9 to 16% is dietary fiber[4-5].Several studies have shown that consumption of both amaranth lipids and protein can lower the serum levels of cholesterol fractions (VLDL-cholesterol and LDL- cholesterol), as well as triacylglycerol in experimental animal species, such as hamsters, rats and chickens that were raised on hypercholesterolemic diets[6-10]. Similar effects were reported in hamsters fed the whole flour[8], or in rabbits fed the extruded whole flour[11]. To our knowledge, however, no study has examined the impact of routine supplementation of amaranth flour, or its products, on the profile of fatty and bile acids in the gut of normal animals.Considering that previous works have evaluated the cholesterol-lowering effect of amaranth in animals consuming cholesterolemic diets[9-12], and in some cases, the protein and/or lipid substitution were not clearly stated[8, 13-14], the objective of the present work was to assess if prolonged supplementation with extruded whole amaranth grain can offer any health value to the normal rat. Therefore, this paper will examine the alterations occurring in the lipid profile of normolipidemic rats fed a normolipidemic diet, in addition to other lipidemic parameters and its impact on the intestinal bile acid profile.
2. Materials and Methods
2.1. Materials
- The amaranth (Amaranthus cruentus, cv. BRS-Alegria, a Brazil adapted grain amaranth cultivar) grains were obtained from Embrapa Cerrados (Centro de Pesquisa Agropecuária dos Cerrados, Planaltina, GO, Brazil) and the flour was produced by grinding in a hammer Mill (Marconi MA580, Piracicaba, SP, Brazil) to pass a 20 mesh screen, and later extruded in a laboratory scale Brabender single-screw extruder (model GNF 1014/2, Duisburg, Germany). The extruder settings were: 18% moisture, 3:1 compression rate, and temperatures of 60-65, 125 and 150℃, for the 1st, 2nd and 3rd zones, respectively. After extrusion, the dry material was submitted to grinding (20 mesh) and storage in double, thick polyethylene bags, at 4℃.
2.2. Animals and Diets
- The experimental protocol was authorized by the Ethics Committee of the University of Campinas (1171-2/2007), which follows the norms of the Brazilian College of Animal Experimentation (COBEA). Forty-eight 21-day old male Wistar rats (CEMIB, University of Campinas) were randomly divided into four groups and housed in individual stainless steel cages and under acclimatized conditions (22º, 12-hr light-dark cycles, 60% relative humidity), with food and water constantly available.Whole amaranth grain was ground and extruded before they were added to the diet. Extrusion is a thermoplastic process of cooking flours in which a combination of a limited amount of moisture and mechanical work produces high attrition, resulting in the evolution of heat, gelatinization of the starches and denaturation of the proteins. Four diets (two containing only casein, and two casein plus amaranth) were prepared according to the American Institute of Nutrition (AIN 93-G)[15], modified to supply either 12 or 18% protein in each case, by manipulation of the corn starch content, and substituting the amaranth flour for part of the standard casein, so as to provide 35% of the final protein content as amaranth protein. The lipid and carbohydrate contents of the modified diets were adjusted accordingly. The diets were made to be isoproteic and isoenergetic at each protein level, and were fed ad libitum for 48 consecutive days. Food intake was obtained by weighing the bowls daily and the mass gain by weighing the animals twice weekly.Feces were collected daily throughout the assay and stored in a freezer for analysis. At 21 days, half (six) of the animals of every group were submitted to 12-hour of fasting, anesthetized with ketamine (80 mg/kg of body weight) and xylazine hydrochloride (8 mg/kg of body weight) for blood collection by heart puncture and organ excision. The remaining animals continued to receive the diets to the end of the assay, when they were submitted to the same procedure of the first half. Liver and cecum were removed from each animal, washed in saline and wiped in gauze prior to weighing. The cecum was frozen together with its contents in liquid nitrogen and stored at –20ºC for the analysis of short-chain fatty acids. Blood samples were collected in Vacuettes® containing EDTA and kept in ice until centrifugation (1000g, 15 min) in order to obtain the plasma, which was immediately stored in criotubes at –20℃ topped with nitrogen gas.
2.3. Blood Analysis
- Plasma lipids were determined by the enzymatic-colorimetric method[16-17] for triacylglycerols by a commercial kit (Laborlab 02700, São Paulo, Brazil). Total and HDL-cholesterol were quantified by the use of diagnostic kits Bioclin K053 and K015 (Santa Branca, Brazil), and the LDL and VLDL-cholesterols estimated using the empirical equation of Friedewald[18]. Serum glucose determination was accomplished by the glucose oxidase-peroxidase method, and insulin by radioimmunoassay[19]. Total serum proteins and albumin were quantified by colorimetric assay, using the biuret and bromcresol-green reactions, respectively (Laborlab 03800, Campinas, Brazil).
2.4. Liver and Fecal Analyses
- Total liver cholesterol was determined following the chromatographic procedure[20]. The method of Bligh and Dyer (1959) was followed for extraction and quantification of the total lipids from the liver and feces. The short-chain fatty acid composition was determined by liquid chromatography[21-22] was employed (Prominenece Shimadzu Co. liquid chromatography, Kyoto, Japan, equipped with a DAD SPD-M20A, and a Luna 5µm, C-18 100Ǻ, 250 x 4.6 mm, column). Bile acids were quantified in the fecal material by gas chromatography (ACME, model 6100, equipped with FID), using both He and N2 as carrier gases[23-25].
2.5. Statistical Analysis
- The data were tabulated as means ± SEM and analyzed using monovariate ANOVA from the SAS program, version 6, followed by the discriminatory Tukey test, with p ≤ 0.05 as the criterion for significance.
3. Results
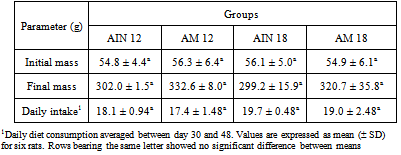
- In general, partial substitution of the standard protein casein by the extruded amaranth, whether at the 12 or at the 18% protein level, resulted in no significant changes of either body mass or diet consumption (Table 1), thus attesting to the general adequacy of the standard diet supplemented with the extruded flour of whole amaranth.
3.1. The Blood and Liver Cholesterol and Lipids
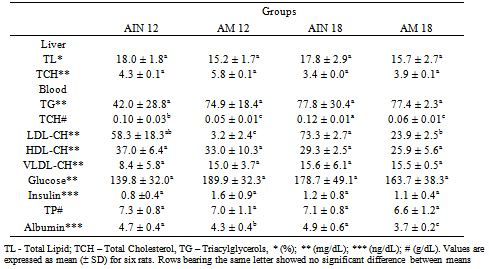
- Consumption of a normolipidemic diet by normolipidemic rats began to show a significant decrease of about 50% in the levels of total serum cholesterol at 20 days of feeding, an effect that was maintained throughout the entire experiment (Table 2) and was evident at both the 12 and 18% protein levels. It was interesting to note, however, that the reduction in total cholesterol was due almost exclusively to the lowering of LDL cholesterol, without affecting the HDL-cholesterol fraction. In spite of the reduction of the serum cholesterol, no alteration was observed in the total hepatic cholesterol content, consistent with the other blood parameters, glucose, insulin and total serum protein and albumin, as shown in Table 2.Additionally, the normal blood triacylglyerol levels were not affected by the dietary interventions, and also no effects in terms of total lipids were observed in the livers as a result of either the level of protein or the inclusion of amaranth in the diets.
|
|
|
3.2. Fecal and Intestinal Parameters
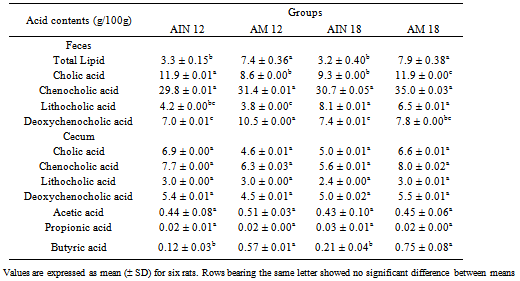
- Although the amount of fecal lipids did not exhibit any alteration as a result of increasing the level of either of the dietary proteins, from 12 to 18%, the substitution of casein for amaranth in the diet did produce an outstanding two-fold elevation of the total output of fecal lipids. Such an increase could be partly consistent with the five to six-fold increase in butyric acid noticed in the cecum contents against no alteration in the levels of either the acetic or propionic acids. It was remarkable, however, that the increase of butyric acid in the cecum appeared not to depend on the level of dietary protein (Table 3).
4. Discussion
- Regarding the effect of the diet modification on the fecal bile acids, some differences were observed that were related to both the protein level and the type of protein. Excretion of the primary bile acid, cholic acid, was diminished in both the AIN-18 and AM-12 groups, compared to the other two diets, whereas no alteration was found in the contents of the other primary acid, chenodeoxycholic acid. Meanwhile, the level of the dietary protein had a clear influence on the secondary acids of the amaranth group, indicating that excretion of lythocholic acid increased by about 100% as the protein content was raised by 50%. Excretion of the undesirable deoxycholic acid, in turn, increased commensurably with the decrease of its precursor, cholic acid, as casein was partially substituted for by the amaranth protein (Table 3). Various studies have reported cholesterol-lowering effects of several of the main fractions of amaranth using hypercholesterolemic experimental animals[6-11] in reducing total serum cholesterol. Hypercholesterolemic rats fed hypercholesterolemic diets for 28 days containing amaranth showed significant decreases of serum total cholesterol and triacylglycerol levels[10]. In the light of our results, however, it is suggested that the same determinant factors or mechanism responsible for the cholesterol-lowering effect in the hypercholesterolemic were also operating in the normocholesterolemic animal yet, without diminishing the HDL-cholesterol levels as has been found in the hypercholesterolemic studies. Likewise, reduction in the levels of serum cholesterol has been observed in chickens[6], hamsters[8] and rats that had their diets supplemented with the oil or squalene isolated from amaranth[7]. Different fractions of the grain, however, have shown varying effects. Hamsters fed a diet prepared with amaranth protein isolate, for instance, exhibited significant reduction in the serum levels of total cholesterol, but this decrease also included an undesirable reduction in HDL-cholesterol[13]. Alternatively, Berger et al. [8] have found that whereas the refined oil, crude oil or flakes do not show significant reductions of cholesterol in hypercholesterolemic hamsters, the intervention did diminish the levels of serum triacylglycerols in the refined oil group.Meanwhile, another study conducted with protein-induced hypercholesterolemic rabbits, which later received a normolipidemic diet supplemented with either extruded or defatted amaranth, or the oil fraction, showed that substantial reductions of total and LDL-cholesterol did occur with the extruded flour than when the oil fraction was used[11]. In our study with normolipidemic, normocholesterolemic rats and diets, however, the reduction in LDL-cholesterol was substantial even with only 35% substitution of the protein. This was most noteworthy because knowing that 50% of the cholesterol is transported by the HDL particles[26], no accompanying reduction of the HDL-cholesterol occurred. These results therefore suggest that routine consumption of a diet with partial substitution of dietary protein by extruded amaranth grain can exert a cholesterol lowering effect without bringing a negative impact on the levels of HDL-cholesterol in normocholesterolemic individuals. Although the amaranth grain has a nutrient composition that makes it potentially hypercholesterolemic, it is still possible that the simple substitution of 35% of the casein with the vegetable protein was partly responsible for the observed effect. Carrol and Kurowska[27] have shown that the mere substitution of casein with another protein, like that of soy, can have an LDL-cholesterol reducing effect in rabbits and rats. Since the extruded amaranth has a methionine/lysine ratio similar to that of soy protein[11], the possibility that this factor be also partly responsible for the overall serum cholesterol reduction of amaranth cannot be excluded. However, the additional effects noticed on the fatty and bile acid profiles could not be attributed to the partial substitution of casein.A greater excretion of fecal lipids was also observed in the group of animals fed the extruded amaranth flour (Table 3), an effect that could be attributed to either the presence of fiber, antioxidant substances, or specific proteins, or even to a combination of two or three of these[9]. This effect has been also observed in mice fed a diet supplemented with quinoa protein and the hypocholesterolemic effect suggesting that the decrease in cholesterol level was associated to both inhibition of synthesis and stimulation of catabolism of cholesterol as well as the inhibition of the re-absorption of bile acids from the intestine [28]. It should be noted that this effect did not influence the normal serum triacylglycerol levels.Dietary fibers have long been recognized as having such physiological properties as a lowering effect of both serum sugar and cholesterol levels[29]. Their intestinal fermentation may give rise to short-chain fatty acids thus favoring a lower intestinal pH, which is associated with a lesser population of pathogenic colonic microbiota, lower solubility of bile acids and an increased absorbability of minerals and a reduction of ammonia absorption from the intestine[30]. The lower colonic pH could also inhibit the enzyme 7α-dehydroxylase, which is responsible for the conversion of primary into secondary bile acids[31]. Among the short-chain fatty acids, the advantages of butyric acid stand out because of its ability to help regulate the process of cell differentiation and to stimulate the immunogenicity of cancerous cells[30-32]. In our study a greater production of butyric acid was found in the groups of animals that received the amaranth diets (Table 3). Inclusion of the amaranth flour in the diets also increased the proportion of dietary fiber and, particularly soluble fibers, which were not present in the control diet. A direct impact on fermentation and short-chain fatty acid generation, mainly butyrate, has been reported to occur from the substitution of the standard cellulose fiber by natural sources of fiber[33-34]. Normally, about 5% of the bile acid pool is removed daily from the entero-hepatic circulation by way of fecal excretion[35]. Considering that cholesterol is a precursor of the primary bile acids, such loss should then represent a net decrease in circulating cholesterol. It appears therefore that the ability of amaranth to promote a higher excretion of both deoxycholic acid and total lipids may be at least partly responsible for the cholesterol lowering effect of this grain. It has also been shown that amaranth, as is the case with quínoa, exhibits in vitro bile-acid binding capacity, especially of the secondary bile acids[28,36]. It should be pointed out, however, that a similar effect has been reported in rats fed diets containing resistant starch from two different bean cultivars[37].Although one study has reported that extruded amaranth can have a high glycemic index in vitro[38], our serum glucose and insulin data showed no detectable adverse response of the rat in the present study.
5. Conclusions
- From the above data it is concluded that chronic consumption of supplementary amounts of extruded amaranth grain by normolipidemic young Wistar rats promoted the decrease of serum total and LDL-cholesterol without affecting the HDL-cholesterol fraction, different from what has been observed in studies with hypercholesterolemic animals. It was also evident that the levels of either serum glucose or insulin were significantly affected by the long term supplementation. Moreover, inclusion of the extruded amaranth in the diet promoted higher excretions of total lipids and of the undesirable secondary bile acid, deoxycholic acid; the latter observation being consistent with the cholesterol-lowering effect observed in the plasma. Finally, no reduction of circulating triacylglycerols below normal levels was detected after chronic consumption of this grain by the normolipidemic rat. Our data support the notion that moderate routine consumption of whole, extruded amaranth does not cause any visible metabolic imbalance and that it is not necessary to substitute the entire dietary protein in order for the normal rat to obtain its potential health benefits. Considering the impact that the more common farinaceous or proteinaceous components of the classical western diet has on human health, the present data obtained in young rats also suggest that further studies should be pursued in young humans. The possibility that judicious or moderate substitution of classical staples for amaranth products in the diet of the young population should redound favorably in better public health is not negligible.
ACKNOWLEDGEMENTS
- The authors acknowledge the kind cooperation of Dr. Carlos Roberto Spehar, of Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) – Cerrados, for the donation of the amaranth grain, and to the Brazilian National Council for Cientific and Technological Development – CNPq for grants 302901/2005 and 134179-2007-2. Thanks are also due to Mrs. Onéida Vasconcelos and to CBO Analítica, Campinas, SP, for the fatty and bile acid determinations.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML