-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(5): 142-152
doi: 10.5923/j.fph.20120205.05
Trends in Particle Formation of Bioactive Compounds Using Supercritical Fluids and Nanoemulsions
M. Thereza M. S. Gomes , Diego T. Santos , M. Angela A. Meireles
LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas)
Correspondence to: M. Angela A. Meireles , LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas).
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This review discusses the recent developments in the application of supercritical fluid technologies for the production of composites or encapsulates of bioactive compounds. Various supercritical particle formation technologies are briefly described, including processes in which the supercritical fluid acts as a solute, solvent, and antisolvent. The main features and mechanisms of antisolvent techniques that contribute to the understanding of the fundamentals of the Supercritical Fluid Extraction of Emulsions (SFEE) process are described. The published literature on SFEE, including the results and perspectives of its application in various industrial fields, are discussed. This article is the first comprehensive review specifically focused on the formation of particles using the SFEE technique.
Keywords: SAS, SFEE, Supercritical, Bioactive Compounds, Novel Processing Techniques
Article Outline
1. Introduction
- Particle formation and encapsulation technologies are widely employed in the pharmaceutical, cosmetic, and food industries. Examples of classical micronization processes include spray drying, spray chilling and spray cooling; extrusion coating; fluidized bed coating; liposome entrapment; coacervation; inclusion complexation; centrifugal extrusion and rotational suspension separation[1]. However, all of these techniques have inherent limitations.Supercritical fluids have been used as solvents, solutes, and antisolvents for micro- and nanoparticle formation in a variety of compounds and have overcome all of the limitations of the traditional techniques. These limitations include poor control of particle size and morphology, degradation of thermosensitive compounds and low encapsulation efficiency[2]. The possibility of obtaining solvent-free microparticulate particles with a narrow size distribution curve using supercritical fluids is very attractive[3]. Supercritical fluids, which were first discovered in 1879, have an exceptional solubility for solids and liquids compared with liquid or gaseous fluids. Variations in the operating conditions to increase the solvation power make this technology a solid option for the recovery of several types of substances. The properties of these fluids have been extensively explored in the extraction and/or separation steps to obtain valuable compounds, such as flavors, colorants, and other biomolecules[4]. A promising new field for supercritical fluids is the formation of particles containing these compounds.The aim of this review is to discuss several of the recent developments in the application of supercritical fluid technologies for the production of composites or encapsulates of bioactive compounds. Bioactive compounds are extranutritional constituents that typically occur in small quantities in nature, are part of the food chain, and have an effect on human health. In this review, the various supercritical particle formation technologies are briefly described. The main features and mechanisms of the antisolvent techniques that primarily contribute to understanding the fundamentals of the Supercritical Fluid Extraction of Emulsions (SFEE) process and the results of and perspectives on applying these techniques in various industrial fields are discussed.
2. Principles of Particle Formation
- Encapsulation has been defined as packaging solid, liquid or gaseous materials into microcapsules that release their contents at controlled rates over prolonged periods of time under specific conditions [5],[6]. The size of the particles formed through encapsulation may be classified as follows: macro (>5000 µm), micro (1.0–5000 µm), and nano (<1.0 µm) [7]. Different morphologies can be obtained depending on the physicochemical properties of the core and wall materials and the encapsulation techniques used during production. In general, the two main structures are mononuclear capsules, which contain one core material enveloped by a carrier material, and aggregates, which consist of many core materials embedded in a matrix of coating material [8],[9].Composites are frequently produced by the simultaneous precipitation of the core and coating materials, which leads to the dispersion of the core material particles into a matrix of coating material. Encapsulates are produced when the coating material is precipitated as a thin shell over a previously existing particle of the core material [10]. Both types are produced for multiple purposes, such as controlling the release of core material in a desired quantity and location, increasing the dissolution rate of slightly water-soluble materials and modifying the surface properties of particles used in pharmaceutics, catalysts, cosmetics, the printing industry and energetic materials [11]. Figure 1 shows the general structures of encapsulates and composites.
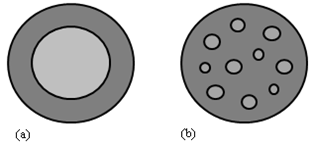 | Figure 1. The structures of (a) an encapsulate and (b) a composite |
3. Particle Formation by Nano- and Microencapsulation
- There are several studies that describe the nano- and microencapsulation technologies that are used to encapsulate bioactive compounds [6], [9], [14-16]. Microencapsulation techniques can be divided into chemical processes, such as molecular inclusion and interfacial polymerization, physicochemical techniques, such as coacervation and liposome entrapment, and physical processes, including spray drying, spray chilling or spray cooling; extrusion; co-crystallization and fluidized bed coating. Additional information on conventional techniques is provided in [5] and [17].There are specific features and characteristics that are disadvantages in each process, such as thermal denaturing, large residual solvent concentrations, and difficulties in controlling particle size and size distribution during processing. These limitations may affect particle stability, flow properties, and delivery efficiency [18]. The use of supercritical fluids as an alternative medium for nano- and microencapsulation can improve the results obtained using conventional techniques. Published reviews indicate that these methods have the potential to overcome the drawbacks previously described [19-23].
4. Nano- and Microencapsulation Using Supercritical Fluids
- Carbon dioxide is the primary fluid applied to produce composite particles using supercritical fluid methods[11] because it enables the process to be performed at near ambient temperatures in an inert atmosphere, which avoids the degradation of the bioactive compounds. The supercritical region can be achieved at moderate pressures and temperatures (Tc = 304.2 K, Pc = 7.38 MPa). A number of modified processes that use supercritical fluids in particle formation have been described in the literature. These processes are classified according to the role of the supercritical fluid in the process, as follows: solvent[Rapid Expansion of Supercritical Solutions (RESS)]; solute[Particles from Gas-Saturated Solutions (PGSS)]; or antisolvent[Supercritical AntiSolvent (SAS)], including its numerous modifications [24]. These classifications are briefly described in the following sections, and additional details regarding these methods can be found in other reviews [10], [11], [19], [23-25].
4.1. Supercritical CO2 as a Solvent
- The first review article on applying the supercritical fluid method in particle design focused on the RESS method, which was the first method used to produce particles [26]. In the RESS process, the substance to be powdered is first dissolved in a supercritical fluid. This mixture is then depressurized through a nozzle, which leads to the rapid precipitation of the dissolved matter as the supercritical fluid vaporizes. The absence of liquid organic solvents, the mild processing temperatures, and the purity of the final product make this process particularly attractive for biomedical applications [27]. Many drugs, such as salicylic acid [28], naproxen [29], ibuprofen [30], griseofulvin and β-sitosterol [31], have been micronized using the RESS technique.
4.2. Supercritical CO2 as a Solute
- The solubility of compressed gases in liquids is generally quite high. Production of particles using the gas-saturated solution (PGSS) process is based on the high solubility of supercritical CO2 in many substances, including molten polymers, oils and fats [32]. The PGSS process consists of solubilizing supercritical CO2 in melted or liquid-suspended substance(s), which leads to a gas-saturated solution that is expanded through a nozzle to form fine particles through precipitation after rapid expansion as a consequence of a drastic reduction in solubility [33]. The PGSS process has been applied in various fields to produce products ranging from inorganic powders to pharmaceutical compounds. Jung & Perrut [19] and [34] list several applications of this method for food and food-related products.Another application of the PGSS process is drying liquid solutions to produce fine powders, or PGSS-drying [34]. Varona et al.[35] recently used PGSS-drying to encapsulate lavandin oil in starches by removing the water from an oil-in-water emulsion stabilized with N-octenyl succinic anhydride (OSA) starches as surfactants.
4.3. Supercritical CO2 as an Antisolvent
- Supercritical antisolvent precipitation is also known as GAS (gas antisolvent), PCA (precipitation by compressed antisolvent), ASES (aerosol solvent extraction system), SEDS (solution enhanced dispersion by supercritical fluids), and SAS (supercritical antisolvent) [36]. These processes are essentially the same, with differences in the feed mode of the solvent and antisolvent, which can be co-current or counter-current, depending on the type of injector used, and can use batch or semi-continuous modes [37].Encapsulation using the SAS technique is based on the same simple principles of the RESS method in which a core material and a carrier are co-precipitated together [2]. This process is well known and has been applied to several types of compounds, including explosives [38], polymers [39], [40], pharmaceuticals[41], [42], and pigments [43]. The SAS method has been thoroughly reviewed by [36]. The advanced application of supercritical fluids inmicro/nanoencapsulation technology, with the emulsion process referred to as Supercritical Fluid Extraction of Emulsions (SFEE), will be evaluated in this review.
4.4. Supercritical Fluid Extraction of Emulsions (SFEE)
- SFEE combines the emulsion techniques and the SAS processes. Emulsion techniques generally require large quantities of organic solvents, and their removal involves additional separation techniques and the use of high temperatures. In addition, SAS is not able to produce particles within the nanometric scale, and the resulting products have an increased tendency for particle agglomeration [10]. To overcome these disadvantages, Chattopadhyay et al. [44] combined the two technologies and patented a new encapsulation method termed the Supercritical Fluid Extraction of Emulsions (SFEE). This method allows the removal of organic solvents during the process and enables the production of nanoscale particles that improve the solubility of bioactive compounds in aqueous solutions, which increases their bioavailability.The SAS method is based on combining the substance to be micronized or encapsulated dissolved in an organic solvent with a supercritical fluid, which acts as an antisolvent. Upon mixing, the supercritical fluid saturates and depletes the liquid solvent by decreasing its solvation power through extraction, and the solute precipitates as microparticles. If a wall material is also dissolved in the organic solvent, composites or encapsulates are formed by co-precipitation with the solute [10], [19]. The experimental setup and principles of the SFEE process are basically the same as those of SAS, but in SFEE, supercritical CO2 is used as an antisolvent to eliminate the organic solvent from the droplets of an oil-in-water (O/W) emulsion [45]. An O/W emulsion containing the core materials to be precipitated dissolved in its dispersed phase (e.g., a conventional organic liquid solvent) is injected into the precipitation vessel with a CO2 flow rate. The final product is a micro- or nanosuspension of the substance in water. The differences in the SAS and SFEE processes are as follows: (a) in SFEE, an emulsion containing the substance to be precipitated dissolved in its dispersed phase is injected, whereas in SAS, a simple solution of the substances is injected; (b) SFEE requires additional steps to produce a powdery product because an aqueous product is formed; (c) the preparation of the initial materials is more complex in SFEE; and (d) emulsion droplet size distribution is a controlling parameter in addition to the other parameters involved in the SAS process (e.g., pressure, temperature, flow rate, and concentration)[10]. However, the SFEE technology is a promising method for producing nanometer particles of natural substances in water [46]. Narrower size distributions can be produced by SFEE because particle size is strictly related to the droplet size and distribution of the starting emulsion, and particle agglomeration can be prevented by the water/surfactant external phase [47]. Using the same pressure, temperature, and solution flow rate for both the SFEE and SAS methods, Shekunov et al. [45] observed a substantial difference in the resulting size and shape of the particles. SFEE produced prismatic crystals with a volume-weighted diameter typically between 0.5 and 1 µm, whereas SAS produced longer crystal dimensions of between 20 and 200 µm and a volume-weighted diameter above 10 µm. Thus, a 10-fold reduction in the particle size was achieved using SFEE compared with the particles produced using SAS.Mattea et al. [48] described the phenomenon that occurs during the SFEE process by investigating a system composed of a β-carotene + dichloromethane-CO2-water + starch-based surfactant. Each drop of the organic solvent behaved as a miniature gas antisolvent precipitator, and multiple particles formed inside the drop. Depending on the CO2 pressure and temperature, the solubility of CO2 in the aqueous and organic phases changed and caused swelling and shrinking of the drop due to the diffusion of supercritical CO2 into the drop and dichloromethane out of the drop.
4.5. SFEE steps
4.5.1. Emulsion Preparation
- Before initiating the SFEE process, an oil-in-water emulsion must be prepared. In general, these emulsions are prepared with the aid of surfactants. Certain of the surfactant materials used to prepare the O/W emulsion have a double functionality in the SFEE process as both a surfactant to stabilize the emulsion and a coating material in the final dry product [46]. Surfactants also act as protective layers and reduce the agglomeration of the final particles[45],[49]. Mezzomo et al. [50] used a Pluronic F127 surfactant/coating material to encapsulate the extract from pink shrimp residue and observed that the emulsion was not stable due to incorrect Hydrophilic-Lipophilic Balance (HLB) values from the surfactant. The authors then used a modified starch (Hi-Cap 100) to achieve high encapsulation efficiency. Additional research is needed to optimize the effectiveness of SFEE for encapsulation.When using a polymer without emulsification properties as a coating material, such as poly-lactic-co-glycolic acid (PLGA), surfactants are only used to stabilize the emulsion. Polyvinyl alcohol (PVA) is the most popular surfactant used in the production of PLGA-stable nanoparticles in the SFEE process. From a food application perspective, the use of food-grade surfactants is important. Studies that have used food-grade surfactants for bioactive compound encapsulation via SFEE are extremely scarce. In the literature, all of the studies are related to the precipitation of carotenoids using a modified starch as the surfactant [46], [48], [50], [51]. Table 1 in section 4.8 lists all of the surfactants that have been tested using the SFEE process.Silva et al. [52] provided an overview of the surfactants used in nanoemulsion production for food applications. The authors focused on nanoemulsion production methods, which are classified as either high-energy or low-energy. There are a number of mechanisms available for the production of emulsions. High-speed stirring mixers [46],[47],[51], high-pressure homogenization [45], [53], [54], and ultrasonication [55-58] have been used to form fine emulsions for use in the SFEE process. Microfluidization is an additional alternative for preparing submicron emulsions. Jafari et al.[59] investigated the efficiency of sonication and microfluidization in the production of nanoemulsions and reported that the microfluidizer produced emulsions with narrower size distributions, whereas sonication was a better option in terms of operation and cleaning. Emulsification is one of the important steps in the SFEE process. An advantage of this process is that growth of the particles is limited by the size of the emulsion droplets [49]. However, stable emulsions are required, and the droplets must be protected against flocculation followed by creaming or sedimentation. Coalescence via collisions and Ostwald ripening, which is a molecular diffusion degradation, are the primary reasons for instability in nanoemulsions. Additional details regarding the principles of the formation and stabilization of nanoemulsions are provided in a review article by Tadros et al. [60].Abismaïl et al. [61] reported that smaller average drops can be obtained using ultrasound. Ultrasound requires less surfactant, consumes less energy and produces emulsions that are less polydispersed and more stable compared with the emulsions produced by mechanical processes. Furlan et al. [57] studied the influence of sonication duration on the final particle size distribution. The authors concluded that the duration of sonication slightly influenced the average particle size but had a strong influence on the particle size distribution.
4.5.2. SFEE Processing
- The SFEE process can be performed in a batch, semi-continuous or continuous mode using a similar apparatus. In the SFEE batch mode, an aliquot of the emulsion is placed into the precipitation vessel to be processed. In the semi-continuous mode, the aqueous suspension is removed from the bottom of the precipitation vessel when the extraction process is complete. In the continuous mode, the suspension is continuously removed through a needle valve [45], [53], [58]. Chattopadhyay et al. [53] observed that there were no differences in mean particle size and morphology between the batch and continuous modes.
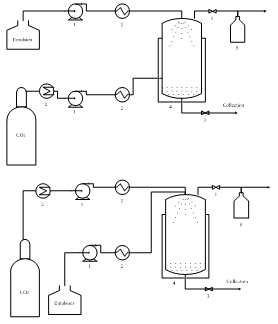 | Figure 2. A schematic diagram of the SFEE apparatus. (A) Counter-current; (B) Co-current; 1, Pump; 2, Heat exchangers; 3, Valves; 4, Precipitation vessel; 5, Flash tank separator |
4.5.3. Elimination of Water
- After SFEE processing, the final product is an aqueous micro- or nanosuspension. Water can subsequently be removed by conventional drying processes, such as spray drying, lyophilization, and microwaving. The high temperature used in most conventional dryers is unsuitable for drying suspensions of bioactive compounds because it accelerates the degradation process. This step can also promote destabilization of the nanoparticles dissolved in water, leading to an increase in the particle size. Santos et al.[51] and Mezzomo et al. [50] spray dried nanosuspensions to produce a dry powder, which increased the size of the particles due to the precipitation of the surfactant during the spray drying process. Most previous studies regarding SFEE did not remove the water, and there is a lack of research evaluating the influence of this step on particle destabilization.
4.6. The Effects of Various Parameters in the SFEE Process
4.6.1. Particle Size
- The effects of various parameters in the SFEE process on precipitate particle size have been evaluated by several authors. No significant changes in particle size have been observed by varying the operating parameters, such as pressure, temperature, processing time and solvent/antisolvent flow rates, in the SFEE process [46], [47], [53]. The literature shows that the primary parameters responsible for particle size control are the emulsion droplet size, solute/solution concentration and organic solvent content in the emulsion [45]. The literature confirms that the particle size is influenced more by the nature of the emulsions than by the mass transfer conditions [47], [53].The literature reports that an increase in organic solvent and polymer concentration alters the particle size. Shekunov et al. [45] observed a reduction in particle size with a decrease in the organic solvent and solute concentrations. According to Chattopadhyay et al. [53] and [54], an increase in the organic solvent concentration in the emulsion can lead to the increased aggregation of the emulsion droplets, resulting in the precipitation of larger particles. Solute and polymer concentrations can be associated with specific functional groups in these compounds, which can change the interfacial tension of the emulsion droplets. The increase in particle size based on the solute concentration is likely due to an increase in the surface tension of the organic solution, resulting in emulsions with larger droplets.In general, an increasing amount of surfactant leads to a decrease in particle size until a minimum value is reached. However, continuously increasing the amount of surfactant in water decreases the polydispersity index of the final product [57]. Kluge et al. [62] studied the effects of PLGA concentrations on the organic droplets at two different emulsion stirring rates and observed that increasing PLGA concentrations led to a higher viscosity of the dispersed organic phase, which favors the formation of larger droplets during emulsification. The authors also observed that the average particle size decreased with an increased emulsion stirring rate, whereas the particle size distributions generally became narrower [47], [53].
4.6.2. Stability of the Emulsion
- The stability of the emulsion is related to the interfacial tension. If the interfacial tension increases as a result of a mass transfer of CO2 to the drop, the emulsion becomes destabilized. Emulsion destabilization also occurs during the depressurization step due to the intense stirring caused by CO2 release from the organic phase [46], [48]. Contact between the emulsion and CO2 to achieve precipitation through the antisolvent effect must occur over a short period of time to minimize the possibility of emulsion destabilization prior to precipitation. However, the elimination of the remaining organic solvent may be slower because emulsion destabilization is no longer an issue after the particles have been produced [46].Varona et al. [35] observed that the stability of the emulsion is drastically reduced when the pressure is increased. Although temperature has a minor effect, stability is related to the creaming effect. According to Chattopadhyay et al. [53], high temperatures and pressures can affect the stability of the emulsion by altering the surfactant-organic phase interactions. In general, a high concentration of surfactant increases the stability of the emulsion [63].
4.6.3. Elimination of Solvent
- The operating pressure and temperature conditions are selected to facilitate the maximum extraction of the organic phase of the emulsion with minimal loss of the solute and polymer due to dissolution in CO2 and to avoid the loss of any emulsion that may wash out in the CO2 stream [47],[53]. Mattea et al. [48] concluded that at pressures below the critical point pressure of CO2-solvent mixtures, the swelling caused by CO2 can be overcome by the diffusion of the solvent out of the drop due to the lower solubility of CO2 in water. Thus, shrinking of the drop can be observed over time.Chattopadhyay et al.[53] and Della Porta & Reverchon[47] observed an increased organic solvent extraction rate with an increased CO2 flow. The efficiency of the solvent extraction increased with pressure, which was basically independent of the solution flow rate under the conditions investigated by Shekunov et al. [45].
4.6.4. Encapsulation Efficiency
- The effectiveness of SFEE in encapsulation can be associated with several parameters, such as polymer type, solute concentration and emulsion formation[50]. Other factors that can influence the encapsulation efficiency include the nucleation rate of the compounds, the size of the formed particles, and the interactions between the carrier material and the solute[51]. Higher solute solubility in the antisolvent can result in a higher loss of the solute, which decreases the encapsulation efficiency due to dissolution in the antisolvent + organic solvent flow. The CO2 flow rate is directly related to the rate of solvent extraction from the emulsion droplet and solute/carrier material losses [47], which have a significant effect on the encapsulation efficiency [51]. Santos et al. [51] observed that a high emulsion flow rate resulted in high encapsulation efficiency, whereas an increased concentration of the surfactant/carrier material led to decreases in encapsulation efficiency. No significant changes were observed by varying the pressure.
4.7. Limitations to the SFEE Process
- The most obvious drawback of SFEE is that the resulting suspension is an aqueous product instead of dry particles. Additional steps are required to produce a powdery product, which can lead to an increase in particle sizes due to agglomeration.This technique has only been applied in the precipitation of solid solutes. Martín et al. [32] suggested the use of SFEE as a possible alternative for the production of compounds extracted from micelles loaded with essential oils, which can have a high viscosity. Another limitation of this technique is that it is only suitable for the encapsulation of hydrophobic compounds. Kluge et al. [62] observed that solvent extraction from an emulsion, similar to the SFEE process, is not ideal for the encapsulation of hydrophilic compounds.In the SFEE process, it is not possible to operate in a completely miscible zone. Due to the nature of the emulsion, there is additional resistance to the transport of CO2 into the organic solvent droplet produced by the water from the emulsion. Different parameters, such as the droplet size when exiting the mixers and the design of the mixer, can affect the feasibility of the process [49]. Figure 3 represents a decision-making diagram for evaluating whether the SFEE process can be applied to encapsulate the solute of interest. This diagram must be considered based on certain conditions because temperature and pressure play an important role during the process. As shown in Table 1, the temperature and pressure for the SFEE process selected in most of the studies were 45°C and 8 MPa, respectively. These conditions were selected based on the solubility of the solute in the solvent and antisolvent. Consequently, the maximum extraction of the organic phase of the nanoemulsion with minimum solute and carrier material losses due to dissolution in CO2 render the process viable [51]. It is important to note that when using a solute in solid form, the final product is a micelle system with powder inside at ambient temperature, but when using a solute with a viscous component that needs to be dissolved in an organic solvent to be pumped, the viscous compound is in the core of the micelle.
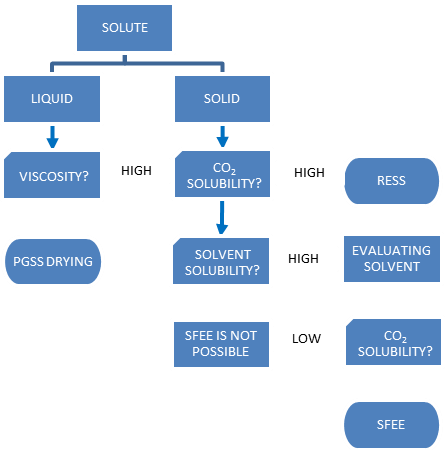 | Figure 3. Diagram of a decision-making tree for the SFEE process |
4.8. Applications
- A variety of active pharmaceutical and food ingredients have been processed using the SFEE process. Reported applications of SFEE are presented in Table 1. Shekunov et al.[45] evaluated the SFEE method for the production of micro- and nanoparticles of cholesterol acetate, griseofulvin and megestrol acetate utilizing both batch and continuous processing for drug delivery applications. Chattopadhyay et al.[53] successfully fabricated composite micro- and nanoparticles using a model system consisting of indomethacin, ketoprofen, and the biodegradable polymers poly(lactic/glycolic) acid and Eudragit RS to form composite particles ranging between 100 and 200 nm in size. SFEE has been used to produce nanoparticles of water-insoluble drugs combined with lipids for pulmonary delivery [54]. The compounds lysozyme (hydrophilic) and ketoprofen (hydrophobic) have been incorporated in poly-lactic-co-glycolic acid (PLGA) using the SFEE process [55],[62]. These authors investigated the phase equilibrium established between ketoprofen and PLGA to further underline the potential of SFEE to serve as a viable manufacturing technique and to highlight a novel application opportunity for this process [56]. In the SFEE processing of PLGA, variations in the PLGA concentration and stirring rates during the preparation of the emulsion have produced particles of pure PLGA with average sizes ranging between 100 nm and several µm with very narrow size distributions. PLGA has been used as a drug delivery system for magnetite nanocrystals stabilized by ricinoleic acid via SFEE[57]. Della Porta & Reverchon [47] evaluated the effectiveness of the supercritical extraction of CO2 from the oil phase of oil-in-water emulsions to obtain spherical PLGA/piroxicam nanostructured microspheres. This process was described as occurring very rapidly due to the enhanced mass transfer of supercritical CO2, resulting in the precipitation of microparticles with a narrower particle size distribution and preventing droplet coalescence or aggregation. Mayo et al.[58] demonstrated that the SFEE process allowed high actual loading of pDNA (19.7%, w/w), a high loading efficiency (>98%), and low residual solvents (<50 ppm) in preparing gene delivery nanoparticles.
5.Conclusions and Perspectives
- From a scientific point of view, particle design using the SFEE process is an attractive option due to the possibility of obtaining solvent-free particles with a narrow size distribution curve in addition to avoiding the degradation of thermosensitive compounds. The concept of using SFEE in an industrial context is currently under development. The primary factor limiting this process is that the final product is a suspension of the desired compound in water. The pharmaceutical industry represents a major focus for particles produced using SFEE technology.Although SFEE has not been widely used for food applications, recent studies applied the technique to the formation of particles from carotenoids, which are an important class of bioactive compounds. Additional bioactive compounds and core materials must be explored in the near future. The results of these researches will have a positive impact in public health: (1) if the target compounds are drugs then, due to increased efficacy smaller amounts of drugs will be needed to treat illness or (2) if bioactive compounds are the target substances then we can expect that new foods can be formulated incorporating these particles.
ACKNOWLEDGEMENTS
- Maria Thereza M. S. Gomes would like to thank the CNPq (process 140641/2011-4) for a doctoral fellowship. Diego T. Santos would like to thank the FAPESP (process 10/16485-5) for a postdoctoral fellowship. The authors acknowledge financial support from CNPq and FAPESP.
References
| [1] | Bernard F. Gibbs, Selim Kermasha, Inteaz Alli, Catherine N. Mulligan, Encapsulation in the food industry: a review, Taylor & Francis Group, International Journal of Food Sciences and Nutrition, vol. 50, no.3, pp. 213–224, 1999. |
| [2] | Diego T. Santos, M. Angela A. Meireles, Carotenoid pigments encapsulation: fundamentals, techniques and recent trends, Bentham Open, The Open Chemical Engineering Journal, vol.4, pp.42–50, 2010. |
| [3] | Ireneo Kikic, Michele Lora, A thermodynamic analysis of three-phase equilibria in binary and ternary systems for applications in rapid expansion of a supercritical solution (RESS), particles from gas-saturated solutions (PGSS), and supercritical antisolvent (SAS), American Chemical Society, Industrial & Engineering Chemistry Research, vol.36, no.12, pp.5507–5515, 1997. |
| [4] | Camila G. Pereira, M. Angela A. Meireles, Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Springer Science, Food and Bioprocess Technology, vol. 3, no.3, pp.340–372, 2010. |
| [5] | Kashappa G. H. Desai, Hyun J. Park, Recent developments in microencapsulation of food ingredients, Taylor & Francis Group, Drying Technology, vol.23, no.7, pp.1361–1394, 2005. |
| [6] | Claude P. Champagne, Patrick Fustier, Microencapsulation for the improved delivery of bioactive compounds into foods, Science Direct, Current Opinion in Biotechnology, vol.18, no.2, pp.184–190, 2007. |
| [7] | Seid M. Jafari, Elham Assadpoor, Yinghe He, Bhesh Bhandari, Encapsulation efficiency of food flavours and oils during spray drying, Taylor & Francis Group Drying Technology, vol. 26, no.7, pp.816–835, 2008. |
| [8] | Peter M. M. Schrooyen, Roelof van der Meer, Cornelis G. De Kruif, Microencapsulation: its application in nutrition, Proceedings of the Nutrition Society, vol.60, no.4, pp.475–479, 2001. |
| [9] | San S. Kuang, Jorge C. Oliveira, Abina M. Crean, Microencapsulation as a tool for incorporating bioactive ingredients into food, Taylor and Francis Group, Critical Reviews in Food Science and Nutrition, vol.50, no.10, pp.951–968, 2010. |
| [10] | María J. Cocero, Ángel Martín, Facundo Mattea, Salima Varona, Encapsulation and co-precipitation processes with supercritical fluids: fundamentals and applications, Elsevier, The Journal of Supercritical Fluids, vol.47, no.3, pp.546–555, 2009. |
| [11] | Masoud Bahrami, Sima Ranjbarian, Production of micro- and nano-composite particles by supercritical carbon dioxide, Elsevier, The Journal of Supercritical Fluids, vol.40, no.2, pp.263–283, 2007. |
| [12] | Fereidoon Shahidi, Xiao‐Q. Han, Encapsulation of food ingredients, Taylor & Francis Group, Critical Reviews in Food Science and Nutrition, vol.33, no.6, pp.501–547, 1993. |
| [13] | Suresh Neethirajan, Digvir S. Jayas, Nanotechnology for the food and bioprocessing industries, Springer Science, Food and Bioprocess Technology, vol.4, no.1, pp.39–47, 2011. |
| [14] | Amparo L. Rubio, Rafael Gavara, Jose M. Lagaron, Bioactive packaging: turning foods into healthier foods through biomaterials, Elsevier, Trends in Food Science & Technology, vol.17, no.10, pp.567–575, 2006. |
| [15] | Job Ubbink, Jessica Krüger, Physical approaches for the delivery of active ingredients in foods, Elsevier, Trends in Food Science & Technology, vol.17, no.5, pp.244–254, 2006. |
| [16] | Mary A. Augustin, Yacine Hemar, Nano- and micro-structured assemblies for encapsulation of food ingredients, The Royal Society of Chemistry, Chemical Society Reviews, vol.38, no.4, pp.902–912, 2009. |
| [17] | Atmane Madene, Muriel Jacquot, Joël Scher, Stéphane Desobry, Flavour encapsulation and controlled release – a review, Institute of Food Science and Technology Trust Fund, International Journal of Food Science and Technology, vol.41, no.1, pp.1–21, 2006. |
| [18] | Daniel J. Jarmer, Corinne S. Lengsfeld, Theodore W. Randolph, Manipulation of particle size distribution of poly(L-lactic acid) nanoparticles with a jet-swirl nozzle during precipitation with a compressed antisolvent, Elsevier, The Journal of Supercritical Fluids, vol.27, no.3, pp.317–336, 2003. |
| [19] | Jennifer Jung, Michel Perrut, Particle design using supercritical fluids: Literature and patent survey, Elsevier, The Journal of Supercritical Fluids, vol.20, no.3, pp.179–219, 2001. |
| [20] | Yukiya Hakuta, Hiromichi Hayashi, Kunio Arai, Fine particle formation using supercritical fluids, Elsevier, Current Opinion in Solid State and Materials Science, vol.7, no.4-5, pp.341–351, 2003. |
| [21] | Zeljko Knez, Eckhard Weidner, Particles formation and particle design using supercritical fluids, Elsevier, Current Opinion in Solid State and Materials Science, vol.7, no.4-5, pp.353–361, 2003. |
| [22] | Alireza Shariati, Cor J. Peters, Recent developments in particle design using supercritical fluids, Elsevier, Current Opinion in Solid State and Materials Science, vol.7, no.4-5, pp.371–383, 2003. |
| [23] | Sang -D. Yeo, Erdogan Kiran, Formation of polymer particles with supercritical fluids: a review, Elsevier, The Journal of Supercritical Fluids, vol.34, no.3, pp.287–308, 2005. |
| [24] | Ángel Martín, María J. Cocero, Micronization processes with supercritical fluids: fundamentals and mechanisms, Elsevier, Advanced Drug Delivery Reviews, vol.60, no.3, pp.339–350, 2008. |
| [25] | Jacques Fages, Hubert Lochard, Jean -J. Letourneau, Martial Sauceau, Elisabeth Rodier, Particle generation for pharmaceutical applications using supercritical fluid technology, Elsevier, Powder Technology, vol.141, no.3, pp.219– 226, 2004. |
| [26] | Jean W. Tom, Pablo G. Debenedetti, Particle formation with supercritical fluids – a review, Elsevier, Journal of Aerosol Science, vol.22, no.5, pp.555–584, 1991. |
| [27] | Pablo G. Debenedetti, Jean W. Tom, Xianmin Kwauk, Sang -D. Yeo, Rapid expansion of supercritical solutions (RESS): fundamentals and applications, Elsevier, Fluid Phase Equilibria, vol.82, pp.311–321, 1993. |
| [28] | Michael Türk, Ralph Lietzow, Formation and stabilization of submicron particles via rapid expansion processes, Elsevier, The Journal of Supercritical Fluids, vol.45, no.3, pp.346–355, 2008. |
| [29] | Michael Türk, Dennis Bolten, Formation of submicron poorly water-soluble drugs by rapid expansion of supercritical solution (RESS): results for Naproxen, Elsevier, The Journal of Supercritical Fluids, vol.55, no.2, pp.778–785, 2010. |
| [30] | Ali Z. Hezave, Feridun Esmaeilzadeh, Micronization of drug particles via RESS process, Elsevier, The Journal of Supercritical Fluids, vol.52, no.1, pp.84–98, 2010. |
| [31] | Michael Türk, Peter Hils, Britta Helfgen, Karlheinz Schaber, Hans -J. Martin, Martin A. Wahl, Micronization of pharmaceutical substances by the rapid expansion of supercritical solutions (RESS): a promising method to improve bioavailability of poorly soluble pharmaceutical agents, Elsevier, The Journal of Supercritical Fluids, vol.22, no.1, pp.75–84, 2002. |
| [32] | Ángel Martín, Huu M. Pham, Andreas Kilzer, Sabine Kareth, Eckhard Weidner, Micronization of polyethylene glycol by PGSS (particles from gas saturated solutions)-drying of aqueous solutions, Elsevier, Chemical Engineering and Processing, vol.49, no.12, pp.1259–1266, 2010. |
| [33] | Kullaiah Byrappa, Satoshi Ohara, Tadafumi Adschiri, Nanoparticles synthesis using supercritical fluid technology – towards biomedical applications, Elsevier, Advanced Drug Delivery Reviews, vol.60, no.3, pp.299–327, 2008. |
| [34] | Eckhard Weidner, High pressure micronization for food applications, Elsevier, The Journal of Supercritical Fluids, vol.47, no.3, pp.556–565, 2009. |
| [35] | Salima Varona, Sabine Kareth, Ángel Martín, María J. Cocero, Formulation of lavandin essential oil with biopolymers by PGSS for application asbiocide in ecological agriculture, Elsevier, The Journal of Supercritical Fluids, vol.54, no.3, pp.369–377, 2010. |
| [36] | Ernesto Reverchon, Supercritical antisolvent precipitation of micro- and nano-particles, Elsevier, The Journal of Supercritical Fluids, vol.15, no.1, pp.1–21, 1999. |
| [37] | Renata Adami, Libero S. Osséo, Rainer Huopalahti, Ernesto Reverchon, Supercritical AntiSolvent micronization of PVA by semi-continuous and batch processing, Elsevier The Journal of Supercritical Fluids, vol.42, no.2, pp.288–298, 2007. |
| [38] | Chang -K. Kim, Byung -C. Lee, Youn -W. Lee, Hyoun S. Kim, Solvent effect on particle morphology in recrystallization of HMX (cyclotetramethylenetetranitramine) using supercritical carbon dioxide as antisolvent, Springer Science, Korean Journal of Chemical Engineering, vol.26, no.4, pp.1125–1129, 2009. |
| [39] | Ernesto Reverchon, Giovanna D. Porta, Igor M. Rosa, Pascale Subra, Didier Letourneur, Supercritical antisolvent micronization of some biopolymers, Elsevier, The Journal of Supercritical Fluids, vol.18, no.3, pp.239–245, 2000. |
| [40] | Lei Yang, Jin -M. Huang, Yuan -G. Zu, Chun -H. Ma, Han Wang, Xiao -W. Sun, Zhen Sun, Preparation and radical scavenging activities of polymeric procyanidins nanoparticles by a supercritical antisolvent (SAS) process, Elsevier, Food Chemistry, vol.128, no4, pp.1152–1159, 2011. |
| [41] | Ruggero Bettini, R. Menabeni, Roberto Tozzi, Marco B. Pranzo, Irene Pasquali, Michele R. Chierotti, Roberto Gobetto, Luca Pellegrino, Didanosine polymorphism in a supercritical antisolvent process, Wiley Periodicals, Journal of Pharmaceutical Sciences, vol.99, no.4, pp.1855–1870, 2010. |
| [42] | Ron T. Y. Lima, Wai K. Nga, Reginald B. H. Tan, Amorphization of pharmaceutical compound by co-precipitation using supercritical anti-solvent (SAS) process (Part I), Elsevier, The Journal of Supercritical Fluids, vol.53, no.1-3, pp.179–184, 2010. |
| [43] | Ángel Martín, Facundo Mattea, Laura Gutiérrez, Félix Miguel, María J. Cocero, Co-precipitation of carotenoids and biopolymers with the supercritical anti-solvent process, Elsevier, The Journal of Supercritical Fluids, vol.41, pp.138–147, 2007. |
| [44] | Pratibhash Chattopadhyay, Boris Y. Shekunov, Jeff S. Seitzinger, Robert W. Huff, Particles from supercritical fluid extraction of emulsion, US Patent N° 0026319 A1, 2004. |
| [45] | Boris Y. Shekunov, Pratibhash Chattopadhyay, Jeff Seitzinger, Robert Huff, Springer Science, Nanoparticles of poorly water-soluble drugs prepared by supercritical fluid extraction of emulsions. Pharmaceutical Research, vol.23, no.1, pp.196–204, 2006. |
| [46] | Facundo Mattea, Ángel Martín, Arán M. -Gago, María J. Cocero, Supercritical antisolvent precipitation from an emulsion: β-Carotene nanoparticle formation, Elsevier, The Journal of Supercritical Fluids, vol.51, no.2, pp.238–247, 2009. |
| [47] | Giovanna D. Porta, Ernesto Reverchon, Nanostructured microspheres produced by supercritical fluid extraction of emulsions, Wiley Periodicals, Biotechnology and Bioengineering, vol.100, no.5, pp.1020–1033, 2008. |
| [48] | Facundo Mattea, Ángel Martín, Constantin Schulz, Philip Jaeger, Rudolf Eggers, María J. Cocero, Behavior of an organic solvent drop during the supercritical extraction of emulsions, American Institute of Chemical Engineers, AIChE Journal, vol.56, no.5, pp.1184–1195, 2010. |
| [49] | Facundo Mattea, Ángel Martín, María J. Cocero, Carotenoid processing with supercritical fluids, Elsevier, Journal of Food Engineering, vol.93, no.3, pp.255–265, 2009. |
| [50] | Natália Mezzomo, Esther de Paz, Marcelo Maraschin, Ángel Martín, María J. Cocero, Sandra R.S. Ferreira, Supercritical anti-solvent precipitation of carotenoid fraction from pink shrimp residue: effect of operational conditions on encapsulation efficiency, Elsevier, The Journal of Supercritical Fluids, vol.66, pp.342–349, 2012. |
| [51] | Diego T. Santos, Ángel Martín, M. Angela A. Meireles, María J. Cocero, Production of stabilized sub-micrometric particles of carotenoids using supercritical fluid extraction of emulsions, Elsevier, The Journal of Supercritical Fluids, vol.61, pp.167–174, 2012. |
| [52] | Hélder D. Silva, Miguel Â. Cerqueira, António A. Vicente, Nanoemulsions for food applications: development and characterization nanotechnology for the food and bioprocessing industries, Springer Science, Food and Bioprocess Technology, vol.5, pp.854–867, 2012. |
| [53] | Pratibhash Chattopadhyay, Robert Huff, Boris Y. Shekunov, Drug encapsulation using supercritical fluid extraction of emulsions, Wiley Periodicals, Journal of Pharmaceutical Sciences, vol.95, no.3, pp.667–679, 2006. |
| [54] | Pratibhash Chattopadhyay, Boris Y. Shekunov, Dong -S. Yim, David Cipolla, Brooks Boyd, Stephen Farr, Production of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx system, Elsevier, Advanced Drug Delivery Reviews, vol.59, no.6, pp.444–453, 2007. |
| [55] | Johannes Kluge, Francesco Fusaro, Marco Mazzotti, Gerhard Muhrer, Production of PLGA micro- and nanocomposites by supercritical fluid extraction of emulsions: II. Encapsulation of Ketoprofen, Elsevier, The Journal of Supercritical Fluids, vol.50, no.3, pp.336–343, 2009. |
| [56] | Johannes Kluge, Marco Mazzotti, Gerhard Muhrer, Solubility of Ketoprofen in colloidal PLGA, Elsevier, International Journal of Pharmaceutics, vol.399, no.1-2, pp.163–172, 2010. |
| [57] | Marco Furlan, Johannes Kluge, Marco Mazzotti, Marco Lattuada, Preparation of biocompatible magnetite–PLGA composite nanoparticles using supercritical fluid extraction of emulsions, Elsevier, The Journal of Supercritical Fluids, vol.54, no.3, pp.348–356, 2010. |
| [58] | Aaron S. Mayo, Balamurali K. Ambati, Uday B. Kompella, Gene delivery nanoparticles fabricated by supercritical fluid extraction of emulsions, Elsevier, International Journal of Pharmaceutics, vol.387, no.1-2, pp.278–285, 2010. |
| [59] | Seid M. Jafari, Yinghe He, Bhesh Bhandari, Nano-emulsion production by sonification and microfluidization – a comparison, Taylor & Francis Group, International Journal of Food Properties, vol.9, no.3, pp.475–485, 2006. |
| [60] | Tharwat Tadros, Paqui Izquierdo, Jordi Esquena, Conxita Solans, Formation and stability of nano-emulsions, Elsevier, Advances in Colloid and Interface Science, vol.108-109, pp.303–318, 2004. |
| [61] | B. Abismaïl, Jean P. Canselier, Anne M. Wilhelm, Henri Delmas, Christophe Gourdon, Emulsification by ultrasound: drop size distribution and stability, Elsevier, Ultrasonics Sonochemistry, vol.6, no.1-2, pp.75–83, 1999. |
| [62] | Johannes Kluge, Francesco Fusaro, Nathalie Casas, Marco Mazzotti, Gerhard Muhrer, Production of PLGA micro- and nanocomposites by supercritical fluid extraction of emulsions: I. Encapsulation of lysozyme, Elsevier, The Journal of Supercritical Fluids, vol.50, no.3, pp.327–335, 2009. |
| [63] | Esther de Paz, Ángel Martín, Antonio Estrella, Soraya R. -Rojo, Ana A. Matias, Catarina M. M. Duarte, María J. Cocero, Formulation of β-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant, Elsevier, Food Hydrocolloids, vol.26, no.1, pp.17–27, 2012. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML