-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(5): 137-141
doi: 10.5923/j.fph.20120205.04
Quantification of Acrylamide in Various Belgian Potato Products Using Solid Phase Extraction and Liquid Chromatography Tandem Mass Spectrometry Detection
Caroline Douny 1, Joëlle Widart 2, Guy Maghuin-Rogister 1, Edwin De Pauw 2, Marie-Louise Scippo 1
1University of Liège, CART (Center for Analytical Research and Technology), Analysis of Foodstuffs of Animal Origin, Faculty of Veterinary Medicine, LIEGE, 4000, Belgium
2University of Liège, CART (Center for Analytical Research and Technology), Mass Spectrometry laboratory, Faculty of Sciences, LIEGE, 4000, Belgium
Correspondence to: Caroline Douny , University of Liège, CART (Center for Analytical Research and Technology), Analysis of Foodstuffs of Animal Origin, Faculty of Veterinary Medicine, LIEGE, 4000, Belgium.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Acrylamide (CH2=CHCONH2), a neurotoxic and potentially carcinogenic substance for human health, is in the glare of the spotlights for a few years. This is mostly due to the fact that acrylamide was found worldwide in various heated foodstuffs. Levels reported in the literature vary from 25 to 2000 µg/kg and potato products are considered as containing the highest level in acrylamide. A possible pathway of synthesis of acrylamide is the Maillard reaction between reducing sugars and the amino acid asparagine. The aim of this study was to develop a liquid chromatography/mass spectrometry method to analyse as quickly as possible acrylamide in a variety of Belgian food samples such as potatoes, French fries, crisp bread, coffee, corn-flakes, etc. The sample preparation consisted in a liquid/liquid extraction, a centrifugation, followed by purification with Solid Phase Extraction (SPE). The instruments used were a Waters 2690 Alliance HPLC system coupled to a Micromass Quattro Ultima Platinum triple-quadrupole mass spectrometer. The analysis was performed in MS/MS mode using isotopic dilution technique for quantification. An internal 13C3 labelled standard was added prior to extraction. Quantification in MS/MS mode was calculated by reconstructing the ion current with the most abundant daughter ions for native and 13C labelled standard (ions of m/z 55 and 58).
Keywords: Acrylamide, French Fries, Belgian Potato Products, SPE, LC-MS/MS
Article Outline
1. Introduction
- Since the Swedish researchers put in evidence that there was a health problem with acrylamide (AA) being present in various common foodstuffs at high concentrations[1], acrylamide is among the “hot” topics in the analysts community. In fact, acrylamide is a compound qualified as neurotoxic and “probably carcinogenic to humans” by the U.S. Environmental Protection Agency[2] and the International Agency for Research on Cancer (IARC)[3]. As it is mentioned in numerous articles, acrylamide present in food is formed at high temperatures by the reaction between amino acids and reducing sugars[4,5]. This reaction is also known as the Maillard reaction[4,6-8]. Heat-treated potato products (chips, French fries) are the products containing the highest amounts of acrylamide with approximately 2000 µg/kg when the smallest amount is approximately 25 µg/kg in coffee or bread. Different surveys have already been conducted in different countries showing that acrylamide is widely present in various foodstuffs[9-16] . In 2003, the Scientific institute of public health confirmed that acrylamide was also present on the Belgian market[17]. Since 2002, laboratories all over the world have begun settling analytical methods to detect that compound. Many different approaches have been explored but the most proposed methods were those using mass spectrometry (MS), either coupled to gas chromatography (GC)[18-21] and either to liquid chromatography (LC)[1,18-20,22-25]. There might be a drawback with GC because some GC–MS methods need a derivatization of acrylamide prior to the injection. With regard to the extraction, solid phase extraction (SPE) is the most widespread. However, Soxhlet extraction[25] or Accelerated Solvent Extraction (ASE)[26,27] are also used.The purpose of the present work was to detect the presence of acrylamide in a variety of Belgian foodstuffs with a method using solid phase extraction and LC with tandem mass spectrometry (MS/MS). The sample preparation was modified from one provided during an acrylamide workshop at the Institute of Public Health in Brussels[28]. The optimisation of acrylamide detection and quantification by mass spectrometry will be pointed out below. Some parameters will be discussed such as collision gas pressure, LC column types, matrix-matched or standard calibration. Finally, results of Belgian foodstuffs study will show the overall content of acrylamide.
2. Experimental
2.1. Chemicals and Material
- Acrylamide (CH2=CHCONH2) (99%, electrophoresis grade) and 13C-labelled acrylamide were purchased respectively from Sigma-Aldrich Corporation (St-Louis, MO, USA) and Cambridge Isotopes Laboratories (Andover, MA, USA). Formic acid (min 99%) was from Acros Organics (New Jersey, USA). Hipersolv HPLC water and methanol were commercially available from BDH Laboratory supplies (Poole, England). The filters purchased were hydrophilic single use syringe filters (0.2 µm pore size, Minisart) from Sartorius (Goettingen, Germany). 50 mL Falcon polypropylene graduated conical tube with cap were commercially available from Greiner Bio-One (Germany). The solid-phase extraction (SPE) cartridges used were OASIS HLB (6 mL, LP 500 mg packing) and Bond Elut – AccuCAT (3 mL, 200 mg packing), respectively from Waters corporation (Milford, MA, USA) and Varian Inc. (Harbor City, CA, USA).
2.2. Instruments
- A 2690 Alliance Separation Modules (Waters, Milford, MA, USA) integrated autosampler, solvent delivery system and column heater coupled to a Quattro Ultima Platinum triple-quadrupole mass spectrometer (Micromass, Manchester, UK) were used for LC-MS-MS analysis.
2.3. Standard Solutions
- Stock solutions of acrylamide (1 mg/mL) and 13C-labelled acrylamide (10 µg/mL), used as internal standard, were prepared by dissolving the compounds in methanol. All solutions were stored at 4℃.
2.4. Sample Preparation
- All samples (raw or fried) were then mixed with a Moulinex mixer (Germany). One gram of sample was weighed and transferred into a 50 mL polypropylene graduated conical tube with cap. Then, sample was spiked with internal standard to achieve a final concentration of 125 µg/kg 13C-acrylamide. 10 mL of water were added. The solution was mixed a first time on a Vortex (high speed) during approximately one minute, then approximately 5 minutes on a rotating shaker.The suspension was centrifuged (3700 g, 15 minutes, 5℃). If supernatant was still turbid, the centrifugation was repeated a second time. Then, 6 mL of the supernatant were filtered on a 0.20 µm syringe filter before applying on solid phase extraction (SPE) cartridge. For the SPE clean-up (see figure 1), the OASIS HLB SPE cartridge (Waters) was conditioned under vacuum with methanol (5 mL), and equilibrated with water (5 mL). Then, 5 mL of the filtered sample were loaded on the OASIS HLB SPE cartridge and the extract was allowed to pass completely through the sorbent material. The OASIS HLB SPE was washed with water (2 mL) and the extract was allowed to pass completely through the sorbent material. Then, the cartridge was eluted with 7.5 mL of methanol. The eluent was collected.For the second step of the clean-up, the Bond Elut AccuCAT SPE cartridge (mixed-mode SPE column consisting of a strong cation exchange and a strong anion exchange sorbent packed into one bed, Varian Inc.) was conditioned under vacuum with 5 mL of methanol. Then, the Bond Elut AccuCAT SPE cartridge was loaded with the solution from the previous step and the eluent was collected directly.The cartridge was rinsed with methanol (1.5 mL) which was combined with the eluent from the OASIS. The extract was then evaporated to dryness under N2 at 40℃. Finally, 500 µL of water were added and the solution was transferred into an injection vial.
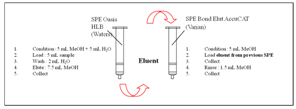 | Figure 1. SPE clean-up |
2.5. LC-ESI-MS-MS
- For analytical separation, an Alltima HP C18 amide column (250 x 2.1 mm I.D., 3 µm, Alltech, Deerfield, IL, USA) and Alltima HP C18HL (250 x 2.1 mm I.D., 3 µm, Alltech, Deerfield, IL, USA) were tested. The elution mode was isocratic using water with 0.1 % acetic acid as LC solvent. The flow-rate was 0.2 mL/min and the injection volume was 20 µL. The total run time was 8 min. The column and samples temperatures were set to 40℃ and 10℃, respectively.
|
2.6. Quantification
- Quantification was possible with isotopic dilution with acrylamide 13C as internal standard (125 ppb). A 6 point-curve in standard solutions was injected: 0, 50, 100, 250, 500 and 1000 ppb.
2.7. Potato Samples
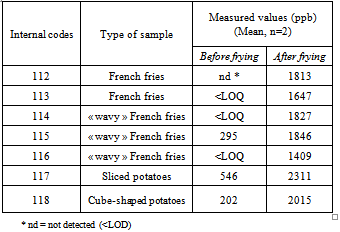
- Samples used for optimising the method were purchased in different supermarkets in Liège, Belgium.Then, the optimised method was applied to potato samples of different kinds provided by a specific Belgian industry: normal and “wavy” French fries, cube-shaped and sliced potatoes. Those samples (internal codes from 112 to 118) were prepared industrially with different amounts of sugar for the reason that they were destined for diverse countries. All samples were analysed before and after frying, which was realised in a deep fat fryer with beef tallow, at 180℃. The fat used was analysed at three defined times: before first sample was fried, in the middle of the series of samples and after last sample.Quality Controls (QC) have been realised with commercially available Belgian raw potatoes.
3. Results and Discussion
3.1. Collision Gas Pressure
- In order to reach a good sensitivity for acrylamide, the collision gas pressure was optimized. Acrylamide is a low mass molecule so the fragmentation is difficult to be achieved with the traditional value of 2.5 10-3 mbar. Six injections of the same solution were done at five pressure values: 1.5x10-3, 7.5x10-3, 1.6x10-2, 3.1x10-2 and 2.4x10-2 mbar.
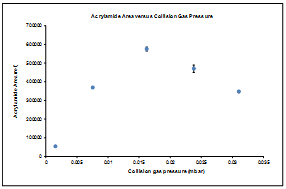 | Figure 2. Acrylamide peak area (transition 72>55) versus collision gas pressure (in mbar) (n=6 for each pressure value, mean ± SD) |
3.2. LC Columns
- Two different LC columns were tested (Alltech Alltima HP C18HL 3µm and Alltech Alltima HP C18 amide 3µm) using the same solutions and analytical protocol. Tests were realised on raw potatoes spiked with a 250 ppb acrylamide solution and 125 ppb internal standard solution. The chromatograms obtained for acrylamide 12C and 13C are shown in Figure 3.
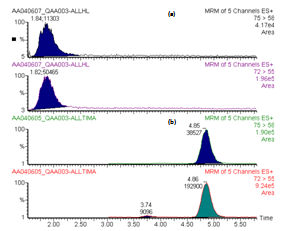 | Figure 3. Acrylamide peaks eluated with (a) Alltech Alltima HP C18HL 3µm column and (b) Alltech Alltima HP C18 amide 3µm column |
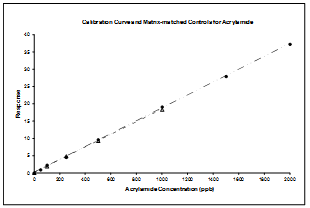
3.3. Matrix-Matched Calibration Versus Standard Calibration
- In order to check matrix effects, we compared the results of a calibration curve realised with standards solutions at different concentrations with the results of raw potato samples spiked at different concentrations. Those samples were used as quality controls. The concentration of acrylamide (in ppb) versus the response is shown in Figure 4. The standard solutions and the spiked samples are represented by dots and triangles respectively. Figure 4 shows that the response obtained with each potato sample matches the values on the calibration curve realised with the standard solutions.There is no significant difference between a calibration curve realised with or without matrix. From now, this will allow using curves realised without matrix and then the analysis will need less samples, less solvents and materials and therefore induce lower costs.Limit of quantification (LOQ) has been established at 50 ppb and limit of detection (LOD) at 25 ppb.
3.4. Real Samples Study
- The optimised method was applied to potato samples. All of them were analysed before and after frying.Results of the content in acrylamide detected in the potato samples are shown in Table 2.The concentrations obtained are in concordance with the values found in literature. What’s more, the results of the three analyses of frying oil were all negative, meaning that the amount of acrylamide found in a sample is coming neither from potatoes previously fried, nor from the oil itself. It seemed that the quantity of sugar present in a variety of French fries is not correlated to the concentration of acrylamide formed during frying.
|
4. Conclusions
- A simple and rapid method for the quantification of acrylamide in a variety of foodstuffs has been developed. The optimisation was done focusing on the collision gas pressure and on the analytical column. This method has been tested with success on different Belgian products (potatoes, French fries, bread…).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML