-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(4): 113-118
doi: 10.5923/j.fph.20120204.06
Impact of Fruit and Vegetables on Oxidative Status and Lipid Profiles in Healthy Individuals
Sapwarobol Suwimol 1, Luangcharoenkul Pimpanit 2, Metavee Aporn 2, Singlaw Pichita 2, Seawsiri Ratiyaporn 2, Jiamjarasrangsi Wiroj 3
1Nutrition and Dietetics Program, Faculty of Allied Health Science, Chulalongkorn University, Bangkok 10330, Thailand
2Undergraduate Program in Nutrition and Dietetics, Faculty of Allied Health Sciences, Chulalongkorn University, 10330, Bangkok, Thailand
3Department of Preventive and Social Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
Correspondence to: Sapwarobol Suwimol , Nutrition and Dietetics Program, Faculty of Allied Health Science, Chulalongkorn University, Bangkok 10330, Thailand.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
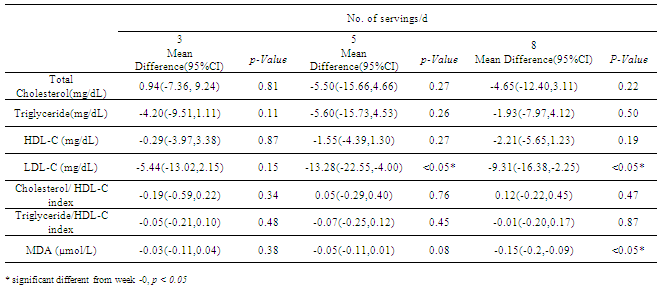
This study aims to investigate the effect of different amount of low, medium and high (3, 5 and 8 servings/day) fruit and vegetables (F+V) consumption on blood lipid profile and plasma malondialdehyde (MDA) in healthy individuals. In a parallel trial, 63 subjects were randomly assigned into one of the three different dietary interventions. Anthropometric, blood lipid profile and MDA were evaluated at baseline and after 4 weeks intervention. The results showed that consumption of F+V 5 and 8 servings/d could significantly reduce low density lipoprotein (LDL-C) concentrations with a mean difference (95% CI) of -13.28 , (-4.00, - 22.55) and -9.31(-2.25, -16.38) mg/dL respectively. In addition, 8 servings/d of F+V consumption had shown to significantly reduced plasma MDA concentrations with a mean difference (95%CI) of -0.15 (-0.09, -0.2) mg/dL, and p-value <0.05. Therefore, consumption of 8 servings F + V daily pose health benefits on both reducing LDL-C concentrations and improving oxidative status.
Keywords: Blood Lipid Profiles, Oxidative Status, Fruits and Vegetables
1. Introduction
- The protective role of regular consumption of fruit and vegetables (F+V) against several chronic diseases such as coronary heart disease[1-4], cancer[5], diabetes mellitus[6], and diverticulitis[7] has been established. In light of the evidence linking fruit and vegetables intake and chronic diseases mentioned, vitamin C, beta-carotene, vitamin E and phytonutrients contained in F+V have been shown a protective health benefit by decreasing malondialdehyde (MDA) level leading to a reduction of inflammatory-related diseases risk[8-10]. In addition, a soluble fiber in diet rich in F+V may assist to optimize serum cholesterol level, a well-known risk factor for cardiovascular disease[11]. The World Health Organization (WHO) therefore recommends that a minimum of 400 g or 5 servings of F + V should be consumed daily in order to obtain health benefits[7]. All nations have been called to establish campaigns to increase fruit and vegetables consumption to meet the WHO recommendations. In respond to WHO recommendations, The Ministry of Public Health of Thailand recommends a minimum of 5 servings or 400 g of F+V each day. Even though the recommendation has been widely promoted, an average F+V consumption in Thai population remains very low compare to the recommendation. The 2003 Thai Health Survey revealed the average F+V consumption in Thai population to be only 268 and 283 g/day in adult males and females, respectively[12].The objective of this study was to investigate the effects of different amounts of F+V intake: low (3 servings/day), medium (5 servings/day) and high (8 servings/day) on blood lipid profile and a marker of oxidative stress among healthy individuals.
2. Methods
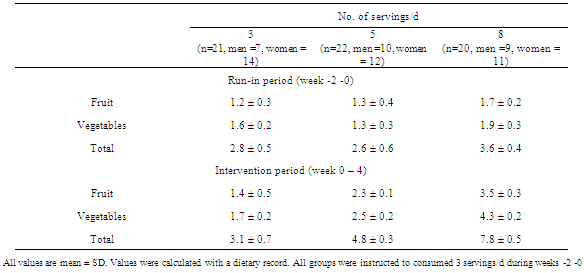
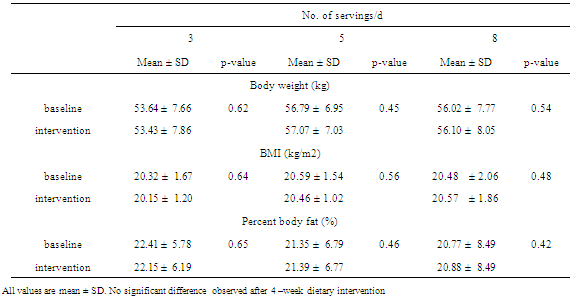
- SubjectsEighty- four healthy individuals aged 18-30 were recruited for the study through advertisements posted at Chulalongkorn University and a nearby community. All participants were in apparent good health as evaluated by medical history, physical examination and screening history. The eligibility criteria included (1) normal serum cholesterol level (less than 200 mg/dL) (2) not receiving medications or dietary supplements known to alter antioxidant levels; (3) no clinical history of cardiovascular, diabetes mellitus, hypertension or inflammatory diseases; (4) no alcohol intake; (5) no recent history of smoking. (6) no known dietary restrictions/food allergies. Eighty-four subjected were recruited to the study. Fourteen subjects reported antioxidant-related supplement used. Seven subjects dropped out because of personal reasons. Sixty-three subjects fulfilled the inclusion criteria and completed the study. (Figure 1).The study protocol was approved by the Ethical Review Committee for Research Involving Human Research Participants, Health Science Group, Chulalongkorn University. Written informed consent was obtained from all subjects prior to enrolling to the study. Subject’s anonymity was preserved. Study designThis trial was a nutritional intervention controlled by registered dietitian from the Department of Nutrition and Dietetics of the Chulalongkorn University. The study was a randomized parallel trial of 4-weeks intervention with a 2-week run-in period. During run-in period, all subjects were assigned to consume 3 servings/d of F+V to diminish the impact of background diet on study outcomes. After a 2-week run-in period, a group of 63 subjects were then randomly assigned into one of the three dietary intervention groups; low (3 servings/day), medium (5 servings/day) and high (8 servings/day) F+V intake. Dietary treatmentSubjects were requested to consume F+V according to their assigned amounts of a low (3 servings/day), medium (5 servings/day) and high (8 servings/day) for four weeks (Figure 1). The energy requirements of the subject were estimated on the basis of body weight, age, and physical activity. Macronutrients composition including carbohydrate, protein and fat was kept similar among the three intervention groups. Registered dietician coached all subjects on F+V serving size to ensure the amounts consumed. Food model was utilized to guarantee serving size understanding. Subjects were allowed to consume F+V according to their cooking preferences. F + V are recommended to eat fresh if possible. In order to evaluate and monitor the subject’s compliance, all subjects were requested to record the F+V consumed at least 2 days a week, including 1 weekday and 1 weekend day throughout the study. Structured interviews were also utilized through at least 2 telephone calls per week. Beside F+V intake, all subjects were requested to keep their lifestyles and exercise habit unchanged throughout the study.Anthropometrics assessment Weight, Body Mass Index (BMI), and percent body fat were measured at baseline (week-0) and the end of treatment period (week-4). Body weight, and percent body fat mass were assessed using a constant current source with a high frequency current (50kHz, 500μA)-bioelectrical impedance analyzer (BIA) (BC-418 body composition analyzer, TANITA corporation, Tokyo, JAPAN). The subject was requested to dress in light attire and bare feet. The 8 polar electrodes were positioned so that electric current was supplied from the electrodes on the tips of the toes of both feet and the fingertips of both hands, and voltage is measured on the heel of both feet and the thenar side of both hands. Body Mass Index (BMI) was calculated as weight/height2 (in kilograms per square meter).Blood analysisFasting blood samples were also collected at baseline and the end of treatment period and were analyzed in a blind fashion. At the beginning (baseline) and at the end of the treatment period (week 4), 9 ml of overnight fasting venous blood samples was collected from all subjects. Blood was collected in tubes that contained lithium heparin. Plasma was collected after centrifugation at 1500x g for 10 min at 4 degree C. Plasma MDA concentrations were analyzed by spectrophotometer, according to the method of Moore and Robert, 1998[13]. Plasma was mixed with 10% trichloroacetic acid (TCA), then centrifuged at 8000x g for 10 min. A 2-Thiobarbituric acid (TBA) was then added to the aliquots. The supernatant fluid was heated at 95 degree C for 10 min before measuring absorbance by spectrophotometer at 532 nm. Lipid profiles analysis was performed at baseline and after 4-weeks of the study. All of the anti-oxidation capacity and blood lipid profiles analysis have been done at the Nutrition laboratory, Faculty of Allied Health Science, Chulalongkorn University.
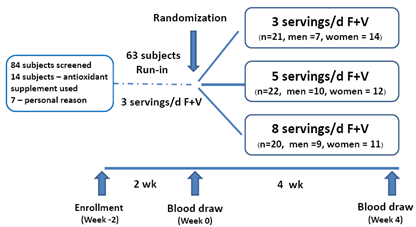 | Figure 1. Study design |
3. Results and Discussion
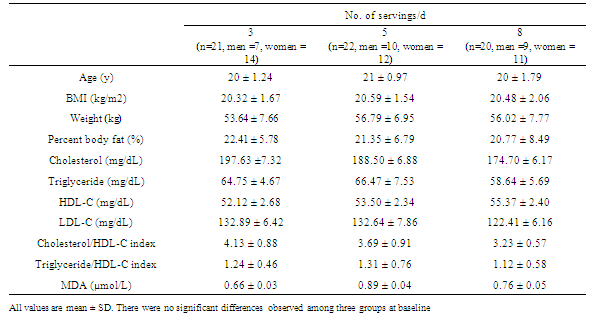
- Sixty-three subjects completed the study with full compliance. Subjects were randomly assigned into one of the three groups; low (3 servings/day), medium (5 servings/day) and high (8 servings/day) F+V. Group of F+V 3, 5 and 8 servings/day consists of 21 (7 men, 14 women, age 20 ± 1.24 years, BMI 20.32 ± 1.67 kg/m2), 22 (10 men, 12 women, age 21 ± 0.97 years, BMI 20.59 ± 1.54 kg/m2), and 20 (9 men, 11 women, age 20 ± 1.79 years, BMI 20.48 ± 2.06 kg/m2) subjects respectively. Baseline (week-0) characteristics of each groups including age, body weight, BMI, percent body fat, total cholesterol, LDL-C, HDL-C, triglyceride, cholesterol/HDL-C index, triglyceride/HDL-C index and plasma MDA are shown in Table 1. There were no significant differences on these baseline characteristics (Table 1).
|
|
|
4. Conclusions
- The outcome of this nutritional trial shows that F+V consumption at least 5 serving/d appears to be effective in improving blood lipid profiles in young healthy adults. The supply of antioxidant components naturally found in F+V could be benefit in improving oxidative status as well. Therefore, including F + V in a minimum of 8 servings/d in a habitual diet may pose health benefit on both reducing LDL-C concentrations and improving oxidative status.
ACKNOWLEDGEMENTS
- This work was supported by staffs at Nutrition laboratory, Faculty of Allied Health Science, Chulalongkorn University. We are grateful for a very helpful of all participants in this project.
References
| [1] | L. Dauchet, P. Amouyel, J. Dallongeville, Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 136, 2588-93, 2006. |
| [2] | P. Knekt, A. Reunanen, R. Jarvinen, R. Seppanen, M. Heliovaara, A. Aromma, Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 139, 1180-9, 1994. |
| [3] | M. R. Law, J. K. Morris, By how much does fruit and vegetable consumption reduce risk of ischemic heart disease? Eur J Clin Nutr 52, 549-56, 1998. |
| [4] | S. Sasazuki, Case-control study of nonfatal myocardial infraction in relation to selected foods in Japanese men and women. Jpn Circ J 65, 200-6, 2001. |
| [5] | The World Cancer Research Fund and American Institue for Cancer Research. Food and Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington, DC: ACIR, 2007. |
| [6] | E. S. Ford, A. H. Mokdad, Fruit and vegetable consumption and diabetes millitus incidence among US adults. Prev Med 32, 33-9, 2001. |
| [7] | FAO/WHO. Diet, Nutrition and the Prevention of Chronic Diseases. Report of A joint FAO/WHO Expert Consultation. Geneva: World Health Organization, 2003. |
| [8] | S. O. Keli, M. G. L. Hertog, E. J. M. Feskens, D. Kromhout, Dietary flavonoids, antioxidant vitamins and incidence of stroke: the Zutphen Study. Arch Intern Med 154, 637-642, 1996. |
| [9] | M. G. L. Hertog, E. J. M. Feskens, P. C. H. Hollman, M. B. Katan, D. Kromhout, Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342, 1007-1011, 1993. |
| [10] | K. A. Steinmetz, J. D. Potter, Vegetables, fruit and cancer prevention: a review. J Am Diet Assoc 96, 1027-1039,1996. |
| [11] | M. Klerk, M. C. J. F. Jansen, P. Van’t Veer, F. J. Kok, Fruit and vegetables in chronic disease prevention. Wageningen, The Netherlands: Grafisch Bedrijf Ponsen&Looijen BV. 1998. |
| [12] | The 3rd health status surveys in Thai population, Health Information System Development Office, Ministry of Public Health; 2003. Available atwww.hiso.or.th/hiso/HealthReport/report2546-2547.php, Cited: July 22, 2011. |
| [13] | K. Moore, L. J. Roberts. Measurement of lipid peroxidation, Free Radic Res 2, 659-671, 1998. |
| [14] | R. B. Singh, S. S. Rastogi, Effect of fat-modified and fruit- and vegetable-enriched diets on blood lipids in the Indian diet heart study. Am J Cardiol 70(9), 869-874, 1992. |
| [15] | D. J. A. Jenkins, D. G Popovich, Effect of a diet high in vegetables, fruit, and nuts on serum lipids. Metabolism 46(5), 530-537, 1997. |
| [16] | J. W. Anderson, T. F. Garrity, C. L. Wood, Prospective, randomized, controlled comparison of the effects of low-fat and low-fat plus high-fiber diets on serum lipid concentrations. AM J Clin Nutr 56, 887-84, 1992. |
| [17] | R. Aller, D. A. de Luis, O. Izaola, F. La Calle, L. del Olmo, L. Fernandez, Effect of soluble fiber intake in lipid and glucose levels in healthy subjects: a randomized clinical trail. Diabetes Res Ckin Pract 65, 7-11, 2004. |
| [18] | H. H. M. Hermsdorff, K. B. F. Barbosa, A. C. P. Volp, B. Pachau, J. Bressan, M. A. Zulet, J. A. Martinez, Vitamin C and fibre consumption from fruits and vegetables improves oxidative stress markers in healthy young adults. Br J Nutr 7, 1-9, 2011. |
| [19] | A. Olivereira, F. Rodriguez-Artalejo, C. Lopes, The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: sex and body mass index interactions. Eur J Clin Nutr 63, 1345-1352, 2009. |
| [20] | H. J. Thompson, J. Heimendinger, A. Haegele, Effects of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis 20, 2261-6, 1999. |
| [21] | E. M. Holt, L. M. Steffen, Fruit and Vegetable Consumption and Its Relation to Markers of Inflammation and Oxidative Stress in Adolescents. J Am Diet Assoc 109(3), 414-421, 2009. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML