-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(4): 104-109
doi: 10.5923/j.fph.20120204.04
Chemical Characterization and Stability of the Bombacopsis glabra Nut Oil
M. H. Chaves 1, F. D. S. Araújo 1, C. V. R. Moura 1, L. J. Tozetto 2, S. Aued-Pimentel 3, M. S. F. Caruso 3
1Departament of Chemistry, Federal University of Piauí, Teresina , PI, 64049-550, Brazil
2Development Company Vale of São Francisco and Parnaíba, CODEVASF, Brasília , DF, 70830-901, Brazil
3Adolfo Lutz Institute, Division Bromatology and Chemistry, São Paulo, SP, CP 1783, 01059-970, Brazil
Correspondence to: M. H. Chaves , Departament of Chemistry, Federal University of Piauí, Teresina , PI, 64049-550, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The aim of this study was to characterize the Bombacopis glabra nut oil (Malvaceae-Bombacoideae) by the determination of its lipid content and fatty acid composition with emphasis on the cyclopropenoid fatty acids (CPFA). The lipids were obtained by five different extraction conditions:[raw almonds: maceration with ethyl ether (I-MA) and n-hexane (II-MA), both at room temperature, and Soxhlet extraction with hexane for 6 (III-MA) and 12 h (IV-MA) and toasted almond: maceration with hexane at room temperature (V-MA)]. Additionally, the oxidation stability of oil by the Rancimat test and the boiling point by thermal analysis (technical TG / DTG) were evaluated. The oil content ranged from 34.99 (I-MA) to 47.36% (IV-MA); oxidation stability was 4.18 h and the boiling point was 373.37 ℃. It should be noted that results about thermal and oxidative stability are been reported for the first time with respect to Bombacopis glabra nut oil. The major oil constituents were palmitic acid (56.06%) and estercúlico (24.83%). The high percentage of CPFA oil, determined by NMR 1H (26.2 to 30.9%) and GC-FID (26.5%), reinforce that the kernels of this species are not suitable for human consumption.
Keywords: Cyclopropenoid Fatty Acids, Oxidation Stability, Thermogravimetric Analysis
Article Outline
1. Introduction
- Bombacopsis glabra (Pasq.) A. Robyns belongs to the Malvaceae – Bombacoideae family, it presents as synonyms Pachira glabra Pasq., Pachira macrocarpa (Schlecht. et Charm.) Walp, Bombax glabrum (Pasq.) A. Robyns and Bombax aquaticum (Aubl.) Schum.[1,2]. This plant is popularly known in Portuguese as “castanha-do-maranhão”, “mamorana”, “castanha-da-praia”, “cacau-do-maranhão” and “cacau-selvagem”. It occurs in tropical and subtropical regions of the America and Europe[3], being native from the Atlantic pluvial forest, from the States of Pernambuco to Rio de Janeiro, as well as in the Amazon, where it grows on the river banks, streams and Amazon River estuary.B. glabra is an arboreal ornamental plant, measuring from 4 to 6 m high. The seeds are the key propagation means, with 100% of germination, which occurs from five to ten days after its seeding. The flowering occurs from September to November, with the fruit maturation at the beginning of the year[3,4]. This species can be used in the recovery of degraded areas, with a good development of the seedlings under full sun, tolerating 30 to 50% shading, moreover, it is used as living stake (“mourão”), by the assorian communities of Santa Catarina Island, Brazil.The fruit contains an average of 18 seeds rich in pleasant flavored oil, consumed by man and wild animals; however, it is still little studied as regards its economical utilization[5]. The annual production is approximately 63 fruit per plant, which corresponds to an estimated 570 kg of seeds per hectare, with a total of 400 individuals[6].Species of the Malvaceae family have as a common characteristic the presence of triacylglycerols of cyclopropenoid fatty acids (CPFA) in their seeds and nuts oil[2]. Cyclopropene ring-containing compounds are associated to several biological effects on animals, including carcinogen and co-carcinogen activities[7-9].The most common cyclopropenoid fatty acids (CPFA) are the malvalic (7-(2-octacyclopropen-1-yl)heptanoic acid) and sterculic (8-(2-octacyclopropen-1-yl)octanoic acid). This last one inhibits the enzyme Δ9-desaturase, which converts the stearic acid into oleic acid, and it is potentially noxious to humans also being able to alter the permeability of the membranes and inhibit the cell reproduction[10].Breyne[6] observed that in some studies on the chemical composition of the B. Glabra oil, the percentage of cyclopropenoid fatty acids (CPFA) ranged from 24.5-34% and, in others there was no mention about the existence of these substances. According to reports in the literature, the divergence in the results of the analysis may be due to the instability of the CPFA, which can be decomposed by heat and acid, and therefore depends on the manner of extraction, the derivatization conditions and the chromatographic analysis[8,9].Regarding the discrepancies on the content of CPFA in B. glabra nuts, the possible undesirable effects related to the consumption of these fruit and the possibility of the Brazilian existing species consist of a genotypically distinct population of plants studied previously, the current work aimed to characterize the Bombacopis glabra nut oil (Malvaceae-Bombacoideae) cultivated in the region of Brasília- DF, capital city of Brazil, by the determination of its lipid content extracted under different conditions. In addition, fatty acid composition with emphasis on the ciclopropenoídicos (AGCP) was determined, as well as evaluated for the first time thermal and oxidation stability of the oil.
2. Materials and Methods
2.1. Instrumentation
- The methyl esters were analyzed on a gas chromatograph with a flame ionization detector (GC-FID), model GC17A, Shimadzu equipped with a SELECT FAME column, Varian, and on a gas chromatograph model GC17A, with a mass detector (GC-MS), model QP5000, Shimadzu, column: SP 2560, SUPELCO. The nuclear magnetic resonance (NMR) analyses were obtained in a Bruker Avance DRX-500 spectrometer and Varian Inova 500, and thethermogravimetric analysis on a TGA-2050 thermogravimetric balance, from TA Instruments. The determination of the stability to oxidation was carried out on the Rancimat Metrohm 743.
2.2. Vegetal Material
- Bombacopsis glabra (Pasq.) Robyns nuts were collected in June 2006, in Brasília-DF, and stored in paper bags at room temperature. The botanic identification was accomplished by Dr. Carolyn Elinore Barnes, from Brasília University-UnB, Brasília, Brazil. A voucher specimen has been deposited in the UnB Herbarium under reference UB 76691.
2.3. Oil Extraction
- B. glabra nut were ground and oil extracted, according to the following procedures: Two samples, of approximately 10 g of nuts, were extracted by maceration. One extract was obtained with 50 mL of ethyl ether (I-MA) and the other one (II-MA) with 50 mL of hexane, for 24 hours at room temperature. Two samples, of nearly 10 g of nuts, were extracted with hexane, on Soxhlet. One sample was extract for 6 hours[11] and the other one, for 12 hours, yielding the extracts III-MA and IV-MA, respectively. The seeds were toasted in a microwave oven at maximum power for two minutes. After the shell removal, 10 g of nuts were ground and submitted to extraction with 50 mL of hexane at room temperature, yielding extract V-MA. The extracts were filtered; the solvent removed in a rotary evaporator at 50℃, and the oil kept in a desiccator until its constant weight.
2.4. Determination of CPFA by 1H NMR
- The 1H NMR spectra was obtained from 50 mg of the oil in natura dissolved into 0.6 mL of CDCl3, using tetramethylsilane (TMS) as an internal reference standard, at the frequency of 500 MHz. The percentage of cyclopropenoid fatty acids was determined using the expression:
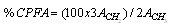 | (1) |
2.5. Determination of Fatty Acid Methyl Ester, Including CPFA, by CG/FID and GC/MS
- The oil extracted with ethyl ether at room temperature (extract I-MA) was transesterified according to the methodology previously described[9,12]. The fatty acid composition was determined by GC-FID and the CPFA confirmed by GC-MS. The compounds were separated on a 100 m capillary column. The analysis conditions by GC-FID were as follows: initial temperature of the column: 45℃ (4 min); heating rate: from 13℃ min-1 to 175℃ (27 min) and from 4℃ min-1 to 215℃ (9 min). Injector and detector temperature: 250℃, carrier gas: hydrogen, flow: 1.9 mL min-1, linear velocity: 34 cm s-1, pressure at the column top: 175 kPa. The quantification was carried out by area normalization and the results expressed in % m/m of methyl esters. The GC-MS analysis were carried out on the following conditions: scan mode; initial temperature of the column: 190℃ (60 min); heating rate: from 10℃ min-1 to 220℃ (30 min), injector and interface temperatures: 225℃ and 230℃, respectively; carrier gas: helium, flow: 0.7 mL min-1, linear velocity: 17.7 cm s-1, split ratio: 1:50 and pressure at the column top: 216 kPa. The mass fragments were obtained by electron impact, with energy of 70 eV, and the analyzer was a quadrupole type. The identification of the components was carried out by comparison with the spectra from the GC-MS library (Nist 62 and Nist 12) and by comparison with the published mass spectra of the methyl esters of the malvalic and sterculic acids 9].
2.6. Thermogravimetric Analysis of the Oil
- The TG/DTG curves of the extracted oil with hexane at room temperature were obtained within a temperature interval of 30-600℃, with a heat ratio of 10℃ min-1, in a nitrogen atmosphere with an output of 50 mL min-1, using an aluminum pot of 20 μL, with an approximately 0.5 mm hole in the lid. The mass loss of the sample was established as the difference between the initial and the final mass. The boiling point (“onset” temperature) was considered as the intersection point of the tangent of the mass loss inclination with the initial base line. The software of the equipment was used to draw the tangent lines and record the boiling temperature[13,14].
2.7. Oxidation Stability Test
- The stability to oxidation was measured on the Rancimat at 110℃ and a constant airflow of 10 L h-1. A sample of 3 g of oil extracted with hexane at room temperature was used. On the Rancimat, the airflow passes through the sample, being bubbled afterwards in a flask containing deionized or distilled water. The airflow carries the volatile carboxylic acids (oxidation outputs), which solubilize and increase water conductibility. The obtained response is an electrical conductibility curve versus time, in which two tangents are built that intercept each other in a point, corresponding, in the time scale, to the induction period or stability to oxidation[15].
2.8. Statistical Analysis
- The analyses were carried out in triplicate, and the averages and standard deviation of the measures were calculated. The ANOVA test was applied, using the Microcal Origin 7.5 to evaluate the difference between the averages of the percentages of CPFA obtained for the samples.
3. Results and Discussion
3.1. Characterization of the Oil
- The B. glabra nuts, after the extraction by means of five experimental procedures, provided the following extracts coded as: I-MA, II-MA, III-MA, IV-MA and V-MA, with oil amounts ranging from 34,99 ± 4.94 to 47.36 ± 0.28% (Table 1). The highest oil yields were obtained from the III-MA (46.28%) and IV-MA (47.36%) samples, which resulted from the extraction of the nuts by Soxhlet for 6 and 12 hours, respectively.The 1H NMR spectra of the oils, obtained by different extraction procedures, presented a singlet in δ 0.77, attributed to the methylenic hydrogens of the cyclopropenic ring[8] and characteristic signals of the structure of triacylglycerols[CH2O: δ 4.15 (dd) and 4.30 (dd); CHO: δ 5.35 (m)][16].
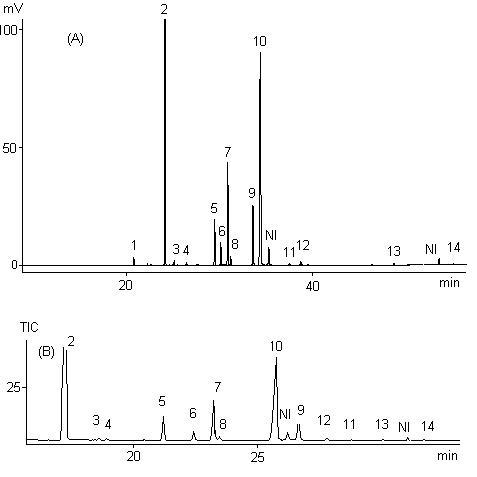 | Figure 1. Chromatograms of methyl esters of fatty acids of B. glabra (castanha-do-maranhão) nut oil obtained in a gas chromatograph: (A) flame ionization detector; (B) mass detector. 1. Myristic acid (14:0); 2. Palmitic acid (16:0); 3. Palmitoleic acid (16:1, 9Z); 4. Margaric acid (17:0); 5. Stearic acid (18:0); 6. Malvalic acid; 7. Oleic acid (18:1, 9Z); 8. Vaccenic acid (18:1, 11Z); 9. Linoleic acid (18:2, 9Z,12Z); 10. Sterculic acid; 11. Linolenic acid (18:3, 9Z,12Z,15Z); 12. Arachidic acid (20:0); 13. Behenic acid (22:0); 14. Lignoceric acid (24:0); NI: unidentified |
| |||||||||||||||||||||||||||||||||||||||||||
3.2. Thermogravimetric Analysis
- The thermogravimetric analysis has shown as a fast technique to measure the boiling point and the vapor pressure of many organic compounds, including vegetal oils or alkyl esters of vegetal oils. According to Goodrum[13], this method does not show any visible evidence that the samples of methyl esters and triacylglycerols of fatty acids decompose before or after boiling.
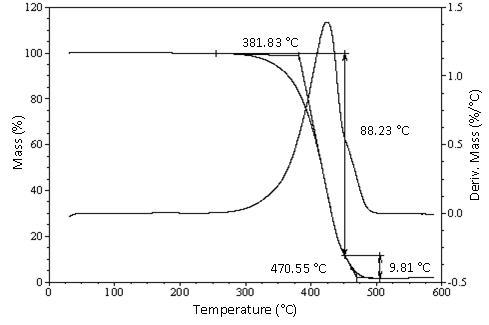 | Figure 2. TG/DTG curve of the B. glabra oil extracted with hexane at room temperature |
3.3. Oxidation Stability
- The B. glabra oil presented an oxidation stability as measured by the Rancimat induction period of 4.18 h, close to the value reported by Ferrari and Souza[23] for sunflower oil (4.47 h). The oxidation stability of an oil is influenced by the polyunsaturated fatty acids, linoleic (18:2) and linolenic (18:3), components of acylglycerols, which derive from the presence in the hydrocarbon chain of bis-allylic methylene groups (CH2 neighboring the double bonds), which are more reactive than the allylic in the reaction with radical formantion by atmospheric oxygen. The products formed in this reaction are organic peroxides that decompose giving rise to minor hydrocarbon chain oxidized compounds, which contribute to the reduction of oxidation stability[24,25].Dewick[10] reports the sunflower oil with a chemical composition ranging from 50 to 70% of linoleic acid (18:2), justifying thus the oxidation stability described by Ferrari and Souza[23]. In the B. glabra oil, the percentage of polyunsaturated fatty acids (18:2 and 18:3) is only 4.93%, therefore, the stability to oxidation of 4.18 h observed suggests it results, chiefly, from the instability of the cyclopropenoid fatty acids (malvalic and sterculic; 26.49%) present in the chemical composition of this species oil.
4. Conclusions
- Nuts of B. glabra presented a high content of lipids (34.99 to 47.36%), although they are not suitable for human consumption due to the presence of harmful substances, such as triacylglycerols of cyclopropenoid fatty acids.The main fatty acids of the triacylglycerols of the B. glabra oil were the palmitic, followed by sterculic, oleic, linoleic and malvalic acids.The boiling point of the oil was 373.37℃, determined by a technique TG/DTG, but under the conditions studied was not detected any event attributed to the degradation of the triacylglycerols of cyclopropenoid fatty acids. However, the oxidation stability was only 4.18 h. It should be emphasized that results about thermal and oxidative stability are been reported for the first time for Bombacopis glabra nut oil.The obtained results represent an important contribution in order to discourage the indiscriminate consumption of the B. glabra nuts, an evidenced fact through the free trade, through the Internet, of the seeds for nourishing purposes.The results confirm that the specie studied is genotypically similar to those reported in other studies even if not reported the presence of cyclopropenoid fatty acids. These components have not probable been recorded due the conditions of analysis not appropriated.Considering that the B. glabra nuts are not suitable for the human nutrition, it is suggested that they be used for other economic purposes, such as the biodiesel production, however, it is necessary a more accurate survey for this applicability.
ACKNOWLEDGEMENTS
- We would like to thank Dr. Carolyn E. Barnes from Brasília University, for the botanical identification of the species; the CENAUREMN/UFC and NuBBE/UNESP- Araraquara city-São Paulo State, for the NMR spectra; LAPETRO-UFPI, for the TG analyses; CNPq, CAPES and FINEP, for the scholarships granted to M. H. Chaves and F. D. S. Araújo, and for the financial support.
References
| [1] | Paula, V. F., Barbosa, L. C. A., Errington, W., Howard, O. W., Cruz, M. P., “Chemical constituents from Bombacopsis glabra (Pasq) A. Robyns: Complete 1H e 13C RMN assignments and X Ray structure of 5-hidroxy-3,6,7,8,4’- pentamethoxyflavone”. Journal of Brazilian Chemical Society, vol. 13, pp. 276-280, 2002. |
| [2] | Nyffeler, R., Bayen, C., Alverson, W.S., Yen, A., Whitlock, B.A., Chase, M.W., Baum, D.A. “Phylogenetic analysis of the Malvadendrina clade (Malvaceae s.l.) based on plastid DNA sequences”, Organisms, Diversity & Evolution, vol. 5, pp. 109-123, 2005. |
| [3] | Pospíšil, F., Hrachová, B. “Bombacopsis glabra (Pasq.) Robyns: a promising oil-bearing crop for the Socialist Republic of Vietnam”, Agricultura Tropica et Subtropica, vol. 20, pp. 127-142, 1987. |
| [4] | Scalon, S. P. Q., Mussury, R. M., Rigoni, M. R., Scalon Filho, H. “Crescimento inicial de mudas de Bombacopsis glabra (Pasq.) A. Robyns sob condição de sombreamento”, Revista Árvore, vol. 27, pp. 753-758, 2003. |
| [5] | Piccolo, A. L. G. “Sobre o fruto, semente e estágios iniciais de desenvolvimento de Bombacopsis glabra (Pasq.) A. Robyns”, Garcia de Orta, Série de Botânica, vol. pp. 1-4, 1981. |
| [6] | Breyne H. “Bombacopsis glabra (Pasquale) A. Robyns (Bombacaceae) espèce utile pour l’élevage et pour l’alimentation humaine”. Tropicultura, vol. 1, pp. 78-85, 1993. |
| [7] | Vickery, J. R. “The fatty acid composition of seed oils from ten plant families with particular reference to cyclopropene and dihydrosterculic acids”, Journal of the American Oil Chemists Society, vol. 57, pp. 87-91, 1980. |
| [8] | Chaves, M. H., Barbosa, A. S., Moita Neto, J. M., Aued-Pimentel, S., Lago, J. H. G. “Caracterização química do óleo da amêndoa de Sterculia striata St Hil. et Naud”, Química Nova, vol. 27, pp. 404-408, 2004. |
| [9] | Aued-Pimentel, S., Lago, J. H. G., Chaves, M. H., Kumagai, E. E. “Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Nauds seed oil”, Journal of Chromatography A, vol. 1054, pp. 235-239. 2004. |
| [10] | Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach, 3nd ed. Jonh Wiley and Sons, New York, 2009. |
| [11] | Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz: Métodos químicos e físicos para análise de alimentos, 4th ed, IMESP, São Paulo, 2005. |
| [12] | Vieira Júnior, G. M., Silva, H. R., Bittencourt, T. C., Chaves, M. H. “Terpenos e ácidos graxos de Dipteryx lacunifera Ducke”, Química Nova, vol. 30, pp. 1658-1662, 2007. |
| [13] | Goodrum, J. W. Volatility and boiling points of biodiesel from vegetable oils and tallow. Biomass and Bioenergy, vol. 22, pp. 205-211, 2002. |
| [14] | Goodrum J. W., Geller, D. P. “Rapid thermogravimetric measurements of boiling points and vapor pressure of saturated medium-and long-chain triglycerides”, Bioresource Technology, vol. 84, pp. 75-80, 2002. |
| [15] | Antoniassi R. “Métodos de avaliação da estabilidade oxidativa de óleos e gorduras”, Boletim do Centro de Pesquisa de Processamento de Alimentos, vol. 19, pp. 353-380, 2001. |
| [16] | Geris, R., Santos, N. A. C., Amaral, B. A., Maia, I. S., Castro, V. D., Carvalho, J. R. M. “Biodiesel de soja – reação de transesterificação para aulas práticas de Química Orgânica”, Química Nova, vol. 30, pp. 1369-1373, 2007. |
| [17] | Mangas, M. B. P., Rocha, F. N., Suarez, P. A. Z., Meneghetti, S. M. P., Barbosa, D. C., Santos, R. B. Carvalho, S. H. V., Soletti, J. I. “Charaterization of biodiesel and bio-oil from Sterculia striata (chichá) oil”, Industrial Crops and Products, vol. 36, pp. 349-354, 2012. |
| [18] | Lima, J. R. O., Silva, R. B., Silva, C. C. M., Santos, L. S. S., Santos Júnior., J. R., Moura, E. M., Moura, C. V. R. “Biodiesel de babaçu (Orbignya sp.) obtido por via etanólica”, Química Nova, vol. 30, pp. 600-603, 2007. |
| [19] | Conceição, M. M., Candeia, R. A., Silva F. C., Bezerra, A. F., Fernandes V. J., Souza A. G. “Thermoanalytical characterization of castor oil biodiesel”, Renewable and Sustainable Energy Reviews, vol. 11, pp. 964-975, 2007. |
| [20] | Queiroga Neto, V., Bora P. S., Diniz Z. N., Cavalheiro, J. M. O., Queiroga, K. F. “Dipteryx lacunifera seed oil: characterization and thermal stability”, Ciência e Agrotecnologia, vol. 33, pp. 1601-1607, 2009. |
| [21] | Araújo, F. D. S., Moura, C. V. R., Chaves, M. H. “Biodiesel metílico de Dipteryx lacunifera: preparação, caracterização e efeito de antioxidantes na estabilidade à oxidação”, Química Nova, vol. 33, pp. 1671-1676, 2010. |
| [22] | Diniz, Z. N., Bora, P. S., Queiroga Neto, V., Cavalheiro, J. M. O. “Aceite de almendra de la semilla de Sterculia striata: caracterización y estabilidad térmica”, Grasas y Aceites, vol. 59, pp. 160-165, 2008. |
| [23] | Ferrari, R. A., Souza, W. L. “Avaliação da estabilidade oxidativa de biodiesel de óleo de girassol com antioxidantes”, Química Nova, vol. 32, pp. 106-111, 2009. |
| [24] | Araújo, J. M. A. Química de Alimentos: Teoria e Prática. 2. ed., Editora UFV, Viçosa, 1999. |
| [25] | Dabdoub, M. J., Brozel, J. L., Rampin, M. A. “Biodiesel: visão crítica do status atual e perspectivas na academia e na indústria”, Química Nova, vol. 32, pp. 776-792, 2009. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML