-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(3): 79-84
doi: 10.5923/j.fph.20120203.04
Evaluation of Microbiological Quality of Raw Milk Produced at Two Properties in the Far West of Santa Catarina, Brasil
Maldaner Nádia 1, Scapin Diane 1, Oro Débora 1, Rossi Eliandra Mirlei 1, 2, 3, 4, 5, 6
1Laboratório de Pesquisa e Diagnóstico em Microbiologia, Departamento de Ciências Biológicas e da Saúde, Universidade do Oeste de Santa Catarina, Rua Oiapoc, 211, Bairro Agostini, 89900-000 São Miguel do Oeste, SC, Brasil
2Laborató
3rio de Microbiologia e Controle de Alimentos, Instituto de Ciê
4ncia e Tecnologia de Alimentos, Universidade Federal do Rio Grande do Sul - ICTA/UFRGS, Av. Bento Gonç
5alves 9500, pré
6dio 43212, Campus do Vale, Agronomia, Porto Alegre/RS, 91501-970 Porto Alegre, RS, Brasil
Correspondence to: Rossi Eliandra Mirlei , Laboratório de Pesquisa e Diagnóstico em Microbiologia, Departamento de Ciências Biológicas e da Saúde, Universidade do Oeste de Santa Catarina, Rua Oiapoc, 211, Bairro Agostini, 89900-000 São Miguel do Oeste, SC, Brasil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Milk is a food that inherently favors microbial growth and due to its characteristics several precautions must be taken to prevent contamination in its production, processing, marketing and consumption, which are routinely subject to changes. The aim of this study was to evaluate microbiological contamination in milk produced at two farms in the Far West of Santa Catarina, before and after the application of Good Manufacturing Practices (GMP). Initially, samples of the milk, surfaces of equipment and utensils for milking, the teats of animals, disinfectants, and water were tested. Next, we conducted training of the farmers in microbiological analysis of milk samples. The analyses included counts of mesophilic aerobes (MA), Staphylococcus coagulase positive (SA), total coliform (TC), and thermotolerant (FC). The methods used for analyses were those described by the Regulation number 62 of August 26th, 2003 published by the Brazilian Ministry of Agriculture and Food Supply (MAPA) that follows methods recommended by the Compendium of Methods for the Microbiological Examination of Foods - APHA. The mean values for MA, SA, TC, and FC in milk obtained before and after the training were, respectively: 4.88 and 3.69 log colony-forming-unit (CFU)/ ml, 3.04 and 2.37 log CFU / ml, 61.19 Most probable number (MPN) and 17.89 MPN/ ml, and 40.26 and 8.71 MPN/ ml. Thus, according to these results, including training in GMP can improve the quality of milk, with immediate results for MA, TC, and CF. But, beyond the procedures employed, the control and prevention of mastitis could help to avoid contamination by SA.
Keywords: Milk, Contamination, Good Manufacturing Practices
Article Outline
1. Introduction
- Milk, being a complex mixture, nutritious, with a high level of water and a pH close to neutral, is highly perishable. It is a product highly conducive to microbial growth, especially bacterial pathogens[1]. Depending on the manipulations it is subject to, milk can have its physical, chemical and biological properties easily altered by the actions of microorganisms. Thus the number of bacteria in milk, directly influences the quality and safety of dairy products[2]. Due to its characteristics, milk deserves special attention in its production, processing, marketing and consumption. Several factors, such as the health of the herd, sterility of the cleaning equipment and utensils used to obtain it, the health conditions of the milking place, the excretion from the udder of an infected animal and quality of water used on the farm, may influence the microbiological quality of milk prod ucts[3-4].Brazil stands out as one of the largest milk producers in the world. However, despite the fact that present production is growing, many milk producers still use non-specialized methods, resulting in raw material of poor quality[5]. The milk contaminated by high levels of bacteria usually becomes unsuitable for further processing since it does not meet the consumer's expectations in terms of health (nutritional value), safety (hygienic quality) and satisfaction (sensory attributes)[6]. The globalization of markets, including the availability of a variety of imported dairy products, has led the Brazilian consumer to become more demanding about the quality and safety of the products offered[7]. The dairy industry has undergone major transformations in an attempt to become more competitive, with benefits to the producer in terms of quality[8]. The parameters physico-chemical, microbiological, hygienic, and sanitation measures have been deployed by the industry to test and verify the quality of milk[9].The pathogens that have been involved in foodborne outbreaks associated with the consumption of milk include Listeria monocytogenes, Salmonella, Campylobacter, Staphylococcus aureus, B. cereus and Costridium botulinum and thermotolerant coliforms, especially Escherichia coli that is the most common contaminant of raw and processed milk[1],[10]. According to Mhone, Matope, Saidi[10], the total count of bacteria also became one of the criteria to evaluate the classification and processing of dairy products. In aiming to meet the need for improvements in the dairy sector, the Ministry of Agriculture, Livestock, and Supply published Normative Instruction No. 51 (IN 51) on September 18, 2002, establishing definitive standards for the quality of refrigerated raw milk commencing in January of 2012[11].In this context, there arose the need to implement changes and improvements in the milk production chain in order to meet the requirements of NI 51. The adoption of quality programs is also aimed at reducing costs in the process, with consequent increases in quality and product differentiation in the marketplace, and in meeting consumer demands. Due to the relevance of the dairy sector for the national economic scenario, and considering the requirements of the major rule concerning federal sanitary legislation (the IN 51), this study aimed to assess the microbiological quality of refrigerated raw milk produced at two properties in the western end of Santa Catarina, and propose applying techniques of Good Manufacturing Practice (GMP) to the manufacturing production process in order to enhance quality and promote continuous improvement of the dairy sector.
2. Materials and methods
2.1. Collection of Samples
- The study was conducted in two farms located in the Far West of Santa Catarina from November 2010 to May 2011. The state of Santa Catarina has great national highlights in the industry livestock and milk production of the major activities in the economy of the states occupying the 5 th place in the national production of bovine milk in the 2010. The far west region of Santa Catarina is responsible for most of that milk production trough small farms.The samples of refrigerated raw milk and water were collected aseptically into sterilized bottles. Samples of disinfectant solution used for the realization of “pre-dipping” (means immersing the teats in disinfectant solution before milking) were aseptically collected 15 minutes after preparation of disinfectant solution in sterilized bottles. However, samples from the surface equipment, utensils, and teats of milking animals were collected with sterile swabs, by friction in the area of the sample. The swabs were dipped and kept in test tubes containing 10 mL of sterile peptone water 0.1%. All samples were transported in isothermal boxes with ice to the Microbiology Laboratory at the University of West of Santa Catarina- UNOESC, for analysis. After mixing of the samples, decimal dilutions were performed by serial transfer of aliquots of 1 mL from sample tubes into tubes containing 9 mL of sterile water and 0.1% peptone (Difco, France), then the microbiological analyzes were performed.
2.2. Data Collection and Microbiological Analyses
- Initially, a profile of each property was prepared by means of a questionnaire that was used for data collection. The first phase of the study aimed to identify points of contamination of milk during milking, and evaluate the microbiological quality of the milk produced on farms with conventional hygienic practices adopted by producers.To achieve this objective, microbiological analyses were carried out on 20 samples of milk and two samples of disinfectants, two samples of the water used in properties, eight samples from the surfaces of teats of animals, and seven samples from equipment surfaces and milking utensils (two samples from milking equipment, two samples from storage tanks of milk samples, two from milk filters, and one from milk sampling of milk storage cans). Subsequently, in the second phase of the study, after GMP techniques were applied in the production process, additional samples were collected, together with the collection and microbiological analysis of 40 samples of refrigerated raw milk, in order to evaluate the effects of adopting GMP.The samples of refrigerated raw milk, disinfectants, and teat surfaces, milking equipment and utensils, were submitted to microbiological analysis for quantification of mesophilic aerobes (MA), Staphylococcus coagulase positive (SA), total coliform (TC), and thermotolerant (FC). Water samples were subjected to microbiological analysis to quantify microorganisms MA, TC and FC, according to the parameters required by Ordinance No. 518/2004 from the Ministry of Health, published on March 25, 2004[12]. All microbiological analyses were performed in triplicate on plates of the same dilution. The methods used for analyses were those described by the Regulation number 62 of August 26th, 2003 published by the Brazilian Ministry of Agriculture and Food Supply (MAPA)[13] that follows methods recommended by the Compendium of Methods for the Microbiological Examination of Foods - APHA[14].The counts of MA were performed using a pour-plate technique. Aliquots of 1 ml of each dilution were placed on sterile petri dishes, followed by the addition of Plate Count Agar (Merck, Germany). Then the plates were gently mixed and incubated at 36 ± 1 ° C for 48 hours. Plates which grew in the range of 25-250 colonies were counted. The results were expressed as CFU / ml.Determinations of TC and FC in refrigerated raw milk and water were performed using Multiple Tube Fermentation. Aliquots of 1 ml of milk samples at dilutions of 100, 10-1 and 10-2, and aliquots of 10 ml, 1 ml and 0.1 ml of the water samples, were transferred to tubes containing 10 ml of broth containing Lauryl Sodium Sulfate (Merck, Germany). The tubes were then homogenised and incubated at 36 ± 1 ° C for 24–48 hours. After incubation, the degree of turbidity was observed, and production of gas from lactose fermentation was evaluated in Durham tubes. From confirm TC aliquots of positive tubes from the previous test were then transferred to tubes containing 10 ml broth Bile 2% Brilliant Green (Merck, Germany), incubated at 36 ± 1 ° C for 24–48 hours to and to confirm CF put into tubes containing 10 mL of EC broth (Merck, Germany) and incubated at 45 ± 0.2 ° C for 24–48 hours. Tubes showing evidence of gas or effervescence when shaken gently were considered positive. From the combination of numbers corresponding to the tubes that had a positive result in each of the confirmatory tests, the numbers of microorganisms of Most Probable Number (MPN) were determined according to the table contained in the IN 62. The results were expressed in MPN/mL for samples of milk, and in MPN/ 100ml for water samples.To count of TC and FC in disinfectants and on teat surfaces, milking equipment and utensils, the pour-plate overlay technique with agar crystal violet neutral red bile (VRBA) (Difco, France) was used. After homogenization, the plates were incubated at 36 ± 1 °C for 24–48 hours. Dilutions on plates which produced in the range of 25–250 colonies were counted and the results were expressed as CFU/ml. To count and confirm TC and FC, three colonies characteristic of each plate of VRBA were inoculated into tubes containing 10 mL Bile 2% Brilliant Green (Merck, Germany), incubated at 36 ± 1 ° C for 24–48 hours, and inoculated into tubes containing 10 mL of EC broth (Merck, Germany) and incubated at 45 ± 0.2 ° C for 24–48 hours. Tubes with gas or effervescence when gently shaken were considered positive. The numbers of SA were determined by inoculating 0.1 ml from each sample, and seeding with the aid of handle Drigalski on the surface of Petri dishes containing Baird-Parker agar (Oxoid, England). The plates were incubated at 36 ± 1 ° C for 30–48 hours. To make the evaluation easier, plates with 25-250 colonies were selected for reading. Typical colonies were counted (colonies with a shiny black opaque halo surrounded by a clear halo or transparent) and atypical colonies (bright gray or black, without halo or with one halo). For confirmation, we selected an average of three colonies of each type (typical and atypical colonies). Then, the selected colonies were transferred to tubes containing broth and agar Brain Heart Infusion (BHI) (Oxoid, England), followed by incubation for 36 ± 1 ° C for 24 hours. From each subculture, the strains were subjected to coagulase testing, catalase, and fermentation of mannitol salt agar.
2.3. Statistical Analysis
- To evaluate the existence of statistical differences between the parameters evaluated before and after the adoption of GMP procedures for handling, the Practice T-Students test was used. The T-Student analysis was used to compare the average counts of two different groups in order to verify statistically significant differences between them. Data analyses were performed on the SPSS (Statistical Package for Social Sciences) release 17.0. A p-value of < 0.05 was considered statistically significant.
3. Results and Discussion
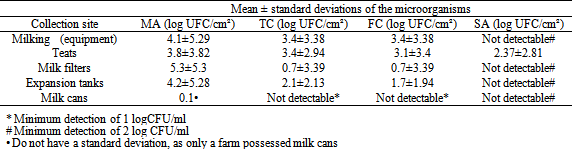
- The main points of contamination by MA, TC, and FC were the milking equipment for the animals and the teats of animals. SA was only found on teats (Table 1). The disinfectant also showed high counts of MA (mean 5.9 log CFU / mL) and TC (mean 3.3 log CFU / ml). Thus, it can be stated that the practice of “pre-dipping”, conventionally used on these properties (reusable towels soaked in a 30% solution of diaminopropil laurilamina) was not effective in reducing microbial contamination of the teats to acceptable levels.In most cases, disinfectants are chosen by habit of use, ease of application, or price, which, coupled with the lack of access to laboratory tests to evaluate the efficacy of the disinfectant solutions used in the routine milking processing, compromise the quality of the milk[15].The water analised at the two properties were in accordance with the standards required for MA contamination by Ordinance No. 518/2004, from the Ministry of Health; however, the samples showed the presence of TC and FC, and may have been a likely source of contamination by TC and FC of teats and milking utensils, and consequently of the milk produced. The result foud results corroborate the statement of Amaral et al.[3], which emphasized the importance of the water used in order to obtain products of good microbiological quality; because, according to the author, the water used in production has great influence on the contamination of the milk, and, being a vehicle for transmission of pathogens, must have characteristics of potability.
| |||||||||||||||||||||||
4. Conclusions
- Training, including adoption of Good Manufacturing Practices, are effective in improving the microbiological quality of refrigerated raw milk, with immediate results in reducing levels of contamination by MA, TC, and CF. Thus, professional monitoring of properties is essential to ensure producers adopt necessary conditions of service to fulfill the definitive parameters of IN 51. However, in addition to adopting these new procedures, control and prevention of mastitis could help to avoid contamination with SA, because this is a major causative agent of infection, and consequently may change the microbiological quality of the milk.
References
| [1] | Chye, F. Y.; Abdullah, A. and Ayob, M. K. (2004). Bacteriological quality and safety of raw milk in Malaysia. Food Microbiology, 21, 535-541. |
| [2] | Arcuri, E. F.; Brito, M. A. V. P.; Brito, J. R. F.; Pinto, S. M.; Angelo, F. F. and Souza, G. N. (2006). Qualidade microbiológica do leite refrigerado nas fazendas. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 58(3), 440-446. |
| [3] | Amaral, L. A.; Rossi, J. O. D.; Nader Filho, A.; Ferreira, F. L. A. and Barros, L. S. S. (2003). Ocorrência de Staphylococcus sp. em água utilizada em propriedades leiteiras do Estado de São Paulo. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 55(5), 620-623. |
| [4] | Angulo, F. J.; LeJeune, J. T. and Rajala-Schultz, P. J. (2009). Unpasteurized milk: a continued public health threat. Clinical Infectious Diseases, 48(1), 93-100. |
| [5] | Correa, C. P. A.; Ribas, M. M. F. and Madrona, G. S. (2009). Avaliação das condições higiênico sanitárias do leite cru em pequenas propriedades do município de Bom Sucesso- PR. Revista Brasileira de Tecnologia Agroindustrial, 3(2), 21-28. |
| [6] | Nanu, E.; Latha, C.; Sunil, B.; Prejit, T. M. and Venon, K.V. (2007). Quality assurance and public health safety of raw milk at the production point. American Journal of Food Technology, 2, 145–152. |
| [7] | Nada, S.; Djekic, I.; Tomasevic, I.; Miocinovic, J. and Gvozdenovic, R. (2012). Implication of food safety measures on microbiological quality of raw milk and pasteurized milk. Food Control, 25, 728-731. |
| [8] | Gonzalez, H. L.; Fischer, V.; Ribeiro, M. E. R.; Gomes, J. F.; Stumpf, J. R. W. and Silva, M. A. (2004). Avaliação da qualidade do leite na bacia leiteira de Pelotas, RS. Efeito dos meses do ano. Revista Brasileira de Zootecnia, 33(6), 1531- 1543. |
| [9] | Guerreiro, P. K.; Machado, M. R. F.; Braga, G. C.; Gasparino, E. and Franzener, A. S. M. (2005). Qualidade microbiológica de leite em função de técnicas profiláticas no manejo de produção. Ciências agrotécnicas, 29(1), 216-222. |
| [10] | Mhone, T. A.; Matope, G. and Saidi, P. T. (2011). Aerobic bacterial, coliform, Escherichia coli and Staphylococcus aureus counts of raw and processed milk from selected smallholder dairy farms of Zimbabwe. International Journal of Food Microbiology, 151, 223–228. |
| [11] | BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Departamento de Inspeção de Produtos de Origem Animal. Instrução Normativa n. 51, de 18 de setembro de 2002. Aprova e Oficializa o Regulamento técnico de identidade e qualidade de leite cru refrigerado. Diário Oficial da República Federativa do Brasil, n. 172, p. 13-22, 20 set. 2002. Seção I. |
| [12] | BRASIL. Ministério da Saúde. Portaria n. 518, de 25 de março de 2004. Estabelece os procedimentos e responsabilidades relativos ao controle e vigilância da qualidade da água para consumo humano e seu padrão de potabilidade. Diário Oficial da República Federativa do Brasil, n. 59; p. 266-270, 26 mar. 2004. Seção I |
| [13] | BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Departamento de Inspeção de Produtos de Origem Animal. Instrução Normativa n. 62, de 26 de agosto de 2003. Oficializa os Métodos Analíticos Oficiais para Análises Microbiológicas para Controle de Produtos de Origem Animal e Água. Diário Oficial da República Federativa do Brasil, p. 14, 18 set. 2003. Seção I |
| [14] | American Public Health Association (APHA) (2001). Compendium of methods for the microbiological examination of foods. 4th ed. Washington: APHA. 676 p. |
| [15] | Medeiros, E. S.; Santos, M. V.; Pinheiro Júnior, J. W.; Faria, E. B.; Wanderley, G. G.; Teles, J. A. A. and Mota, A. A. (2009). Avaliação in vitro da eficácia de desinfetantes comerciais utilizados no pré e pos-dipping frente amostras de Staphylococcus spp. isoladas de mastite bovina. Pesquisa Veterinária Brasileira, 29(1), 71- 75. |
| [16] | Guimarrães, C. P. A. Impacto da Assistência técnica sobre a qualidade do leite. Goiânia. (2008). 82 f. Dissertação (Mestrado em Medicina Veterinária)- Escola de Veterinária, Universidade Federal de Goiás. |
| [17] | Andrade, U. V. C.; Hartmann, W. and Masson, M. L. (2009). Isolamento microbiológico, contagem de células somáticas e contagem bacteriana total em amostras de leite. ARS Veterinária, 25(3), 129- 135. |
| [18] | Fagan, E. P.; Tamanini, R.; Fagnani, R.; Beloti, V.; Barros, M. A. F. and Jobim, C. C. (2008). Avaliação de padrões físico- químicos e microbiológicos do leite em diferentes fases de lactação nas estações do no em granjas leiteiras no Estado do Paraná. Ciências Agrárias, 29(3), 651-660. |
| [19] | Costa, F. F. (2006). Interferência das práticas de manejo na qualidade microbiológica do leite produzido em propriedades rurais familiares. Jaboticabal. 80 f. Dissertação (Mestrado em Zootecnia)- Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista Júlio de Mesquita Filho. |
| [20] | Guido, E. S.; Silva, E. D. P.; Silva, M. C.; Takeuchi, K. P. and Danesi, E. D. G. (2010). Uma abordagem da extensão universitária na melhoria da qualidade do leite na cadeia produtiva do município de Barbosa Ferraz (Paraná). Boletim do Centro de Pesquisa e Processamento de Alimentos, 28(2), 303-312. |
| [21] | Moraes, C. R.; Fuentefria, A. M.; Zaffari, C. B.; Conte, M.; Rocha, J. P. A. V.; Dorneles, A. S.; Silva, P. V.; Corcao, G. and Costa, M. (2005). Qualidade microbiológica de leite cru produzido em cinco municípios do Estado do Rio Grande do Sul, Brasil. Acta Scientiae Veterinariae, 33(3), 259- 264. |
| [22] | Pinto, C. L. O.; Martins, M. L. and Vanetti, M. C. D. (2006). Qualidade microbiológica de leite cru refrigerado e isolamento de bactérias psicotróficas proteolíticas. Ciência e Tecnologia dos Alimentos, 26(3), 645- 651. |
| [23] | Mattos, M. R.; Beloti, V.; Tamanini, R.; Magnani, D. F.; Nero, L. A.; Barros, M. A. F.; Pires, E. M. F.; Paquereau, B. P. D. (2010). Qualidade do leite cru produzido na região do agreste de Pernambuco, Brasil. Semina: Ciências Agrárias. 31(1), 173-182. |
| [24] | Silva, V. A. M.; Rivas, P. M.; Zanela, M. B.; Pinto, A. T.; Ribeiro, M. E. R.; Silva, S. S. P. and Machado, M.. (2010). Avaliação da qualidade físico- química e microbiológica do leite cru, do leite pasteurizado tipo A e de pontos de contaminação de uma Granja Leiteira no RS. Acta Scientiae Veterinariae, 38(1), 51- 57. |
| [25] | Reinemann, D. J. (2003). Review of practices for cleaning and sanitation of milking machines. Bulletin of the International Dairy Federation, 381, 4-18. |
| [26] | Tebaldi, V. M. R.; Oliveira, T. L. C.; Boari, C. A. and Piccoli, R. H. (2008). Isolamento de coliformes, estafilococos e enterococos de leite cru provenientes de tanques de refrigeração por expansão comunitários: identificação, ação lipolílitica e proteolítica. Ciência e Tecnologia dos Alimentos, 28(3), 735-760. |
| [27] | Riekerink, R. G. M. O.; Barkema, H. W.; Scholl, D. T.; Poole, D. E. and Kelton, D. F. (2010). Management practices associated with the bulk-milk prevalence of Staphylococcus aureus in Canadian dairy farms. Preventive Veterinary Medicine, 97, 20-28. |
| [28] | Stamford, T. L. M.; Silva, C. G. M.; Mota, R. A. and Cunha Neto, A. (2006). Enterotoxigenicidade de Staphylococcus spp. isolados de leite in natura. Ciência e Tecnologia dos Alimentos, 26(1), 41-45. |
| [29] | Martins, R. P.; Silva, J. A. G.; Nakazato, L.; Dutra,V. and Almeida Filho, E. S. (2010). Prevalência e etiologia infecciosa da mastite bovina na microrregião de Cuiabá, MT. Ciência Animal, 11(1), 181- 187. |
| [30] | Santana, E. H. W. (2006). Determinação do perigo de consumo do leite cru relacionado à intoxicação estafilocócica. Londrina, 74 f. Tese (Doutorado em Ciência Animal)-Universidade Estadual de Londrina. |
| [31] | NADER FILHO, A.; Ferreira, L. M..; Amaral, L. A.; Rossi Júnior, O. D. and Oliveira R. P. (2007). Produção de enterotoxinas e da toxina da síndrome do choque tóxico por cepas de Staphylococcus aureus isoladas na mastite bovina. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 59(5), 1316-1318. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML