-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(2): 34-39
doi: 10.5923/j.fph.20120202.07
Total Lipid Fractions and Fatty Acids Composition of Low-Fat Beef Burger
Mohamed K. E. Youssef 1, Badway. M. D. Mostafa 2, Magda A. A. Seliem 1, Alyaa M. A. Hashem 2
1Food Science and Technology Department, Faculty of Agriculture, Assiut University, Assiut, 71526, Egypt
2Food Technology Research Institute, Agricultural Research Center, Giza, 12619, Egypt
Correspondence to: Alyaa M. A. Hashem , Food Technology Research Institute, Agricultural Research Center, Giza, 12619, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
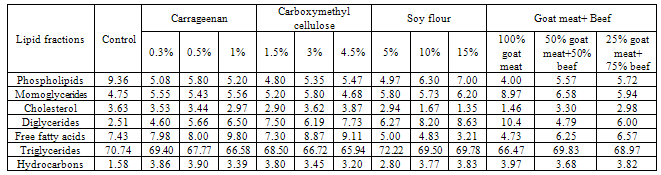
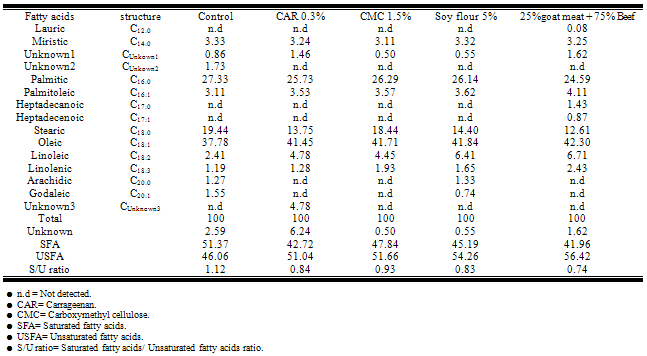
Low-fat beef burgers were produced with four different fat replacer (carrageenan, carboxymethyl cellulose, soy flour and goat meat) which were added at three levels (0.3, 0.5 and 1%), (1.5, 3 and 4.5%), (5, 10 and 15%) and (25, 50 and 100%); respectively. Total lipid fractions were determined by using thin layer chromatography technique for beef burger in both raw and grilled state after 3 months of frozen storage. Data revealed that phospholipids contents were less and triglycerides contents were more in the studied beef burger. Low-fat beef burgers containing 0.3% carrageenan, 5% low-fat soy flour, 1.5% carboxymethyl cellulose, 25% goat meat + 75% beef and control sample (without any fat replacer) gained the highest sensory score, so it was important of determine the fatty acids composition of these samples by the end of frozen storage. Saturated fatty acids were C12:0, C14:0, C16:0, C17:0andC18:0. Predominated, major monounsaturated fatty acids were C16:1, C17:1, C18:1 and C20:1; and the major polyunsaturated fatty acids were descendingly: C18:2, C18:3. Among these fatty acids, the fatty acid C18:1 represented the highest relative percentage of all identified fatty acids.
Keywords: lipid fractions, fatty acids, beef burger, goat meat, fat replacers, low-fat beef burger
Article Outline
1. Introduction
- There had been an increased interest in recent years in ways to manipulate the fatty acid composition of meat. This is because meat is seen to be a major source of fat in the diet and especially of saturated fatty acids, which had been implicated in diseases associated with modern life, especially in developed countries. These include various cancers and especially coronary heart disease. The importance of the link between nutrition and health is a hot topic. Like other food-related sectors, the meat industry is undergoing major transformations, driven among other things by changes in consumer demands[1].The Dietary Guideliness[2] recommended limiting total fat intake to not more than 30% of daily energy intake, with saturated fats no more than 10% and monounsaturated and polyunsaturated fats accounting for at least two- third of daily energy intake. Studies had shown that reduction in fat intake could result in 10% reduction of risk for heart disease, and if persons who are over weight lose weight in addition to modifying their diet, they could lower their risk for cardiovascular heart disease by 20%[3].The fatty acid composition of meat had long been studied but still received a lot of attention in research because of its implications for human health. Besides a lower total fat intake, human nutritionists are recommending a higher intake of polyunsaturated fatty acids [4] and [5].This investigation was carried out in an attempt to clarify the effect of adding fat replacers on total lipid fractions and fatty acids composition of low-fat beef burger after 3 months of frozen storage.
2. Materials and Methods
2.1. Materials
2.1.1. Meat
- 40 kg of fresh lean beef (top round) muscle from old cows (3 years old) were obtained from Assiut slaughter house during March 2010. Visible fat and connective tissue were eliminated manually[6]. The lean beef samples were minced using meat mincer and was used for processing of low-fat beef burger.
2.1.2. Goat meat (Chevon)
- 6.0 kg of fresh meat of goat was obtained from healthy 1-yr-old goat (leg and shoulder cuts) purchased from Assiut local market during March 2010. The goat meat samples were minced using meat mincer and used for processing of low fat-beef burger.
2.1.3. Fat replacers
2.1.3.1. Carrageenan
- 72g of GENUVISCO carrageenan type CSM-2 was obtained from Technogen Company at Dokii, Cairo, Egypt during March, 2010. It is a hydrocolloid consisting mainly of calcium, magnesium, potassium, and sodium sulphate ester of glactose and 3,6 anhydrogalactose co-polymers. It is soluble in cold water exhibiting a viscosity effect almost instantaneously after dispersion. The suspension was prepared by stirring 0.3, 0.5 and 1.0g of carrageenan in 100 ml iced water, then it was added to meat[7].
2.1.3.2. Low-fat Soy Flour
- 1200g of soy flour was purchased from the Food Technology Institute Agriculture Research Center- Giza, Egypt during March 2010. Powdered soy flour was rehydrated (by mixing one part of powdered soy flour with two parts of tap water) before addition to the meat[8].
2.1.3.3. Carboxymethyl Cellulose Sodium Salt (CMC):
- 360 g of carboxmethyl cellulose (CMC) product No: 0385 was purchased from EL- Badry company at Assuit, Egypt during March, 2010. The suspensions were prepared by stirring 1.5, 3.0, 4.5 gm of CMC in 100 ml iced water, then it was added to meat[9].
2.1.4. Spices
- Spices mixture was prepared using equal weights of clove, black pepper, Chinese cubeb, paprika and nutmeg that were obtained from the local market in Assiut during March 2010.
2.1.5. Salt, Onion, Garlic and Parsely
- Salt, fresh onion, garlic and parsley were obtained from the local market in Assiut and used for preparation of beef burger during March, 2010.
2.2. Methods
2.2.1. Preparation of Beef Burger Samples
- The burger formulae were formed using a patty marker (stainless steel model "Form") to obtain round discs 10 cm diameter and 0.5 cm thickness. After preparation of each formulae, the beef burger samples (each sample was ) were packed in polyethelene bags and were stored immediately in a deep freezer at -20℃ for up to three months where samples were required at zero time 1, 2 and 3 months for analyses before and after cooking. Basal beef burger formula included 85.75% minced beef, 1% salt, 0.5% onion, 0.25% garlic, 0.25% parsley, 0.25% mixed spices and 12 ml iced water[7]. The suspensions of carrageenan (0.3, 0.5, 1%), carboxymethyl cellulose (1.5, 3, 4.5%), rehydrated soy flour (5, 10, 15%), and minced goat meat (25, 50, 100%) with different ratios were added to basal formula to prepare different low-fat beef burger treatments.
2.2.2. Cooking of Beef Burger
- The beef burger studied samples were cooked using an electrical grill (Arcelik Mini Firin, Turkey) at 300℃ (the distance between heat source and the sample was 4 cm) for a total of 10 min, 6 min one side and 4 min in the other side [10].
2.2.3. Chemical Analysis
2.2.3.1. Thin layer Chromatography Separation and Identification of Lipids
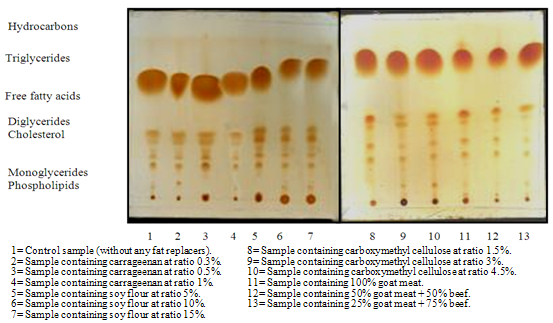
- Total lipids were extracted by chloroform: methanol mixture (2:1 v/v) according to method described by[11] Silica gel G plates (20x20 cm) were used for qualitative and quantitative determination of lipid fractions. Plates were developed in a mixture of petroleum ether, diethyl ether and glacial acid (80: 20: 1, v/v/v). The lipid fractions were visualized by exposure to iodine vapor, the isolated fractions were identified on thin layer plates by comparing their Rf values with that of known lipid standards. For quantitative analysis, the TLC chromatograms were scanned using densitometr modal (Seroscan elvi 146) and the data were analyzed by QS computer program analysis J scans.
2.2.3.2. Determination of Fatty Acids Composition
- ● Preparation of methyl ester of fatty acidsThe methyl esters of fatty acids were prepared from aliquots of total lipids using 5 ml 3% H2SO4 in absolute methanol and 2 ml benzene as mentioned by[12]. The contents were heated for methanolysis at 90℃ for 90 minute and after cooling, phase separation was performed by addition of 2 ml distilled water and methyl ester were extracted with 2 ml aliquots of 5 ml hexane each. The organic phase was removed, filtered through anhydrous sodium sulfate and then concentrated by using rotary evaporator.● Gas liquid chromatography of methyl esters of fatty acidsThe methyl esters of fatty acids were separated using a PYE Unicam Pro-GC gas liquid chromatography with a dual flame ionization, and were carried out on (1.5m x 4mm) SP-2310 column, packed with 55% cyanopropyl phenyl silicone dimensions. Column temperature: At first the temperature was programmed by increasing the temperature from 70-190℃ at the rate of 8℃/, minute. then isothermal for 10 minute at 190℃.The injector and detector temperatures were 250℃ and 300℃, respectively. Carrier gas: nitrogen at the rate 30 ml/minute., hydrogen flow rate 33 ml/minute. and air flow rate 330 ml/minute. The chart speed was 0.4 cm/minute. Peak identifications were established by comparing the retention times obtained with standard methyl esters. The areas under the chromatographic peak were measured with electronic integrator.
3. Results and Discussion
3.1. Total Lipid Fractions of Beef Burger after 3 Months of Frozen Storage.
- The data presented in Table (1) and Figure (1) revealed the total lipid fractions of lipid extracted from beef burgers by the end of frozen storage. The data indicated that, the total lipids of beef burger were fractionated to seven fractions namely: phospholipids, monoglycerides, cholesterol, diglycerides, free fatty acids, triglycerides and hydrocarbons and using thin layer chromatographic technique (TLC). Such data are in good agreement with that previously reported by [13] and [14]. From the data presented in Table (1) it could be noticed that the phospholipids contents were less and triglycerides contents were more in the studied beef burger. Moreover, control sample had the highest contents of phospholipids as compared to other samples. This might be due to high amount of fat content of control samples.
3.2. Total Lipid Fractions of Grilled Beef Burger after 3 Months of Frozen Storage.
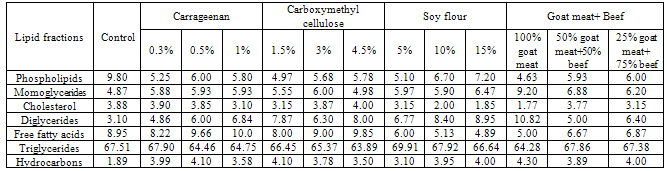
- The data given in Table (2) and Figure (2) indicated that, grilling reduced the content of triglycerides and increased the contents of monoglycerides, diglycerides, cholesterol, free fatty acids and hydrocarbons in all samples. Such results are in agreement with [15] and [13] findings. The increase of phospholipids content was in agreement with[16] findings for cooked beef and pork meat and[17] who reported that the use of dry heat (roasting) induced a slight increase in phospholipids of lamb meat. The decrease of triglycerides content was reported by[14] for roasting chicken meat. Meanwhile, the increase of monoglycerides, cholesterol, free fatty acids and hydrocarbon contents might be occurred during storage which coincide with[13] findings for chicken meat.
3.3. Fatty Acids Composition of Low-Fat Beef Burger
- Data given in Table (3) outlined the fatty acids composition of high and low-fat beef burgers samples which gained the highest sensory score at all the time of storage. The data revealed that saturated fatty acids were C12:0, C14:0, C16:0, C17:0andC18:0. Predominated, major monounsaturated fatty acids were C16:1, C17:1, C18:1 and C20:1; and the major polyunsaturated fatty acids were descendingly: C18:2, C18:3. Among these fatty acids, the fatty acid C18:1 represented the highest relative percentage of all identified fatty acids. The data are in agreement with[18] findings on beef.[19], investigating the fatty acids in beef patties found that the predominating fatty acid in the neutral lipids fraction was the C18:1.
|
 | Figure (1). Total lipid fractions of beef burger after adding fat replacers by the end of frozen storage. |
|
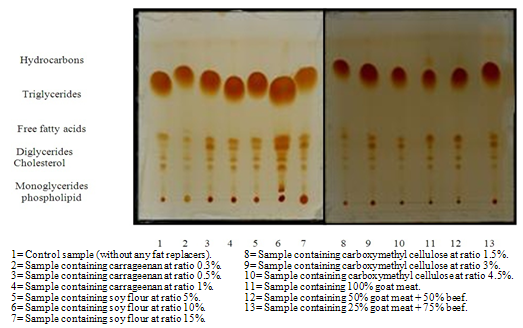 | Figure (2). Total lipid fractions of grilled beef burger after adding fat replacers by the end of frozen storage |
|
4. Conclusions
- On the basis of the above-mentioned data the fatty acids profile of these low-fat meat burgers had a high monounsaturated fatty acids content with oleic acid comprising more than the other fatty acids and this is necessary to decrease cholesterol and the risk of heart disease. The low-fat beef burger recommended to include in diet regimen of both over weight and obese persons as well as diabetic, hyper cholesterolemic and hyperlipidimemic patients.
ACKNOWLEDGEMENTS
- The authors wish to express the acknowledgement for Food Science and Technology Department, Faculty of Agricultural, and Meat and Fish Department, Food Technology Research Institute, Agricultural Research Center for providing the necessary laboratory facilities to accomplish the present work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML