-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(2): 21-27
doi: 10.5923/j.fph.20120202.05
Nutritive and Anti – Nutritive Composition of Chanca Piedra (Stone Breaker)
Gafar M. K. , Itodo A. U. , Senchi D. S.
Department of Chemistry, Kebbi State University of Science and Technology, Aliero, Nigeria
Correspondence to: Itodo A. U. , Department of Chemistry, Kebbi State University of Science and Technology, Aliero, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
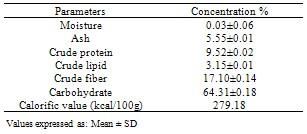
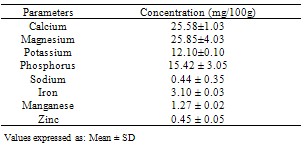
The fresh plants of Chancapiedra collected from Zuru Emirate of Kebbi State, Nigeria were dried, pulverized and subjected to nutritive and anti-nutritive analysis. The proximate composition revealed the presence of Moisture (0.03±0.06% fresh weight), Ash (5.55 ±0.01% dry weight), Crude Lipid (3.15±0.01% dry weight), Crude Proteins (9.52± 0.02% dry weight), Crude fibre (17.10±0.14%), Carbohydrate (64.31± 0.18%) and calorific value of 279.18kcal/100g. The mineral composition revealed include Calcium (25.58±1.03mg/100g), Magnesium (25.85±4.03mg/100g), Potassium (12.10 ± 0.10mg/100g), Phosphorus (15.42±3.05mg/100g), Sodium (0.44±0.35mg/100g), Iron (3.1±0.03mg/100g), Manganese (1.27±0.02mg/100g) and Zinc (0.45±1.05mg/100g). The anti-nutritive compositions are Oxalate (5.34±0.4mg/100g), Phytate (27.58±1.7mg/100g), Hydrogen cyanide (16.10±0.14mg/100g), Nitrate (22.42±0.028mg/100g) and Tannins (15.2± 0.13mg/100g). The results revealed that the plant Chancapiedra contained some essential nutrients.
Keywords: Food, Nuritive, Anti-nutrients, Chancapiedra, Stone Breaker
1. Introduction
- The intake of food and supplementsare utilized in the body for maintenance of good health, growth and energy[1]. A balanced diet mainly consists of macro nutrients, micro nutrients and water. The macronutrients include carbohydrates, fats and proteins whereas the micronutrients are vitamins and minerals. All these are very essential factors for normal functioning of the body[1]. The conventional food plants provides most of these nutrients and they are becoming less available to the middle and lower class people in the society due to the economic constrain and other factors such as increasing population[2], so more people from these classes are now incorporating the non conventional food (wild) plants into their daily mealwhich not only they provide nutrients but also used traditionally for treatment of various ailments[3].As available and cheap as they are thousands of these wild plants are yet to be discovered.Chancapiedrais a wild edible plant belongs to the family ofEuphorbiaceacephyllanthus, and belongs to species ofNiruriamarus. It is synonyms to Phyllanthuscarolinianus, P. sellowianusP. fraternus,P. kirganella,P. lathyroides, Nymphanthusniruri. It is commonly called Chancapiedra in Spain. In Brazil the plant is known as Quebra- pedraorArranca-pedras, carry-me-seed, gale-wind grass, quinine weed which translates to as Stone– breaker[4]. The Hausas of the north Nigeria called itGerontsunsaye.ChancaPiedra is a small, erect, annual herb that grows 30-40cm in height. Itis indigenous to the rainforest of the Amazon and other tropical areas throughout theworld, including Bahamas, Southern India, Africa and China. Phyllanthusniruriis quite prevalent in the Amazon,some tropical areas in Africa and also include some wet rainforest, growing and spreading freely (much like a weed).P. amarus and P. sellowianus are closely related to Phyllanthusniruriin appearance, phytochemical structure and history of use, but typically are found in dry tropical climate of India, Brazil and even Florida and Texas[5].The ChancaPiedraplant has a long history in herbal medicine in every tropical country where it grows. It is employed to similar condition worldwide. The plant is used for the treatment of numerous illness which includes; colic, diabetes, malaria, dysentery fever, flu, tumors, jaundice, and dyspepsia, and also considered as analgesic, and as an aperitif, carminative, digestive, laxative, stomachic, it expels worms, stone from kidneys, increase urination, relieves pain, protect liver, reduces inflammation, treat viral infections, its aids digestion, reduces blood sugar, lowers blood pressure, lowers cholesterol.The natural remedy is usually just standard infusion or weak decoction of the whole plant or its aerial parts[5]. Chancapiedra plant has been subjected to phytochemical research to determine the active constituents and their pharmacological activities, it was revealed that it is a rich source of photochemical including many which have been found only in the Phyllanthus genus many of the active constituents are glycosides, flavonoids, alkaloids, tannins and phenylpropanoids, lipids, sterols and flavones found in the leaf, steam and root of the plant[6]. This plant has shown a promising utilization in the pharmaceutical industries but not much has been done on it nutritional aspect.
2. Materials and Methods
- Sampling and sample treatment: Chancapiedra plants were collected from Zuru local government inKebbi State and identified by a taxonomist at Botany department in Kebbi State University of Science and Technology, Aliero. They were sun dried for threedays and blended into fine powder with a blender machine, sieved and stored in a covered plastic container for further uses. All reagents were of analytical reagent grade unless otherwise stated. Distilled water was used in the preparation of solutions and dilution unless otherwise stated while the proximate composition, mineral composition and anti-nutritive component determinations unless otherwise stated were carried out in triplicates.Proximate analysis: The estimation of the various food parameters inChancapiedra plant was carried out using the following methods.Determination of moisture content: This is a measure of the % moisture lost due to drying at a temperature of 105℃. 2g of the fresh plants of Chancapiedra was weighed (W1) into preweighed crucible (W0) and placed into a hot drying oven at 105℃ for 24 hours. The crucible was removed, cooled in a desiccator and weighed. The process of drying, cooling and weighing were repeated until a constant weight (W2) was obtained andthe weight loss due to moisture was obtained by the equation[6].
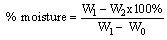 (i)WhereW0 = Weight of the empty crucible, g W1=Weight of fresh sample + empty crucible, g1. W2=Weight of dried sample + empty crucible, g2. Determination of ash content: This is a measure of the residue remaining after combustion of the dried sample in a furnace at a temperature of 600℃ for 3 hours. 2g of the powdered plants sample of Chancapiedrawas weighed (W1) into preweighed empty crucible (W0) and placed into a Lentonfurnace at 600℃ for 3 hours. The ash was cooled in a desiccator and weighed (W2). The weight of the ash was determined by the difference between the powdered leaves sample, preweighed crucible and the ash in the crucible[7]. Percentage ash was obtained by equation ii.
(i)WhereW0 = Weight of the empty crucible, g W1=Weight of fresh sample + empty crucible, g1. W2=Weight of dried sample + empty crucible, g2. Determination of ash content: This is a measure of the residue remaining after combustion of the dried sample in a furnace at a temperature of 600℃ for 3 hours. 2g of the powdered plants sample of Chancapiedrawas weighed (W1) into preweighed empty crucible (W0) and placed into a Lentonfurnace at 600℃ for 3 hours. The ash was cooled in a desiccator and weighed (W2). The weight of the ash was determined by the difference between the powdered leaves sample, preweighed crucible and the ash in the crucible[7]. Percentage ash was obtained by equation ii.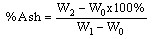 (ii)WhereW0=Weight of empty crucible, gW1=Weight of crucible + powder sample, g W2=Weight of crucible + ash sample, gDetermination of crude lipids: The crude lipid content in the sample of Chancapiedra was extracted using soxhlet extraction.The powder sample (2g) was weighed (W0) into a porous thimble and covered with a clean white cotton wool. Petroleum ether (200cm3) was poured into a 250cm3 extraction flask, which was previously dried in the oven at 105℃ and weighed (W2). The porous thimble was placed into the soxhlet and the rest of the apparatus was assembled. Extraction was done for 5 hours,the thimble was removed carefully and the extraction flask was placed in a water bath so as to evaporate the petroleum ether and then dried in the oven at a temperature of 105℃ to completely free the solvent and moisture. The flask was then cooled in a desiccator and reweighed (W1). The percentage crude lipid was calculated using the equation below
(ii)WhereW0=Weight of empty crucible, gW1=Weight of crucible + powder sample, g W2=Weight of crucible + ash sample, gDetermination of crude lipids: The crude lipid content in the sample of Chancapiedra was extracted using soxhlet extraction.The powder sample (2g) was weighed (W0) into a porous thimble and covered with a clean white cotton wool. Petroleum ether (200cm3) was poured into a 250cm3 extraction flask, which was previously dried in the oven at 105℃ and weighed (W2). The porous thimble was placed into the soxhlet and the rest of the apparatus was assembled. Extraction was done for 5 hours,the thimble was removed carefully and the extraction flask was placed in a water bath so as to evaporate the petroleum ether and then dried in the oven at a temperature of 105℃ to completely free the solvent and moisture. The flask was then cooled in a desiccator and reweighed (W1). The percentage crude lipid was calculated using the equation below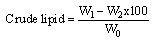 (iii)Where W0=Weight of sample, gW1=Weight of flask + oil, gW2=Weight of flask, gDetermination of crude fiber content: 2g of powder sample of Chancapiedrawas weighed (W0) into a 1 dm3 conical flask. Water (100cm3) and 20cm3 of 20% H2SO4 were added and boiled gently for 30 minutes. The content was filtered through Whatmann No. 1 filter paper. The residue was scrapped back into the flask with a spatula. Water (100cm3) and 20cm3 of 10% NaOH were added and allowed to boil gently for 30 minutes. The content was filtered and the residue was washed thoroughly with hot distilled water, then rinsed once with 10% HCl and twice with ethanol and finally three times with petroleum ether. It was allowed to dry and scrapped into the crucible and dried overnight at 105℃ in an air oven. It was then removed and cooled in a desiccator. The residue was weighed (W1) and ashed at 600℃ for 90 minutes in a Lenton muffle furnace. It was finally cooled in a desiccator and weighed again (W2)6. The percentage crude fiber was calculated using equation viii.
(iii)Where W0=Weight of sample, gW1=Weight of flask + oil, gW2=Weight of flask, gDetermination of crude fiber content: 2g of powder sample of Chancapiedrawas weighed (W0) into a 1 dm3 conical flask. Water (100cm3) and 20cm3 of 20% H2SO4 were added and boiled gently for 30 minutes. The content was filtered through Whatmann No. 1 filter paper. The residue was scrapped back into the flask with a spatula. Water (100cm3) and 20cm3 of 10% NaOH were added and allowed to boil gently for 30 minutes. The content was filtered and the residue was washed thoroughly with hot distilled water, then rinsed once with 10% HCl and twice with ethanol and finally three times with petroleum ether. It was allowed to dry and scrapped into the crucible and dried overnight at 105℃ in an air oven. It was then removed and cooled in a desiccator. The residue was weighed (W1) and ashed at 600℃ for 90 minutes in a Lenton muffle furnace. It was finally cooled in a desiccator and weighed again (W2)6. The percentage crude fiber was calculated using equation viii.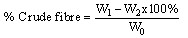 (iv)WhereW0=weight of sample, gW1=weight of dried residue, gW2=weight of ash residue, gDetermination of crude protein content: The crude protein of the Chancapiedrasample was determined using the micro – Kjeldahlmethod[8]. The principle of this method is based on the transformation of protein and that of the other nitrogen containing organic compounds, other than nitrites and nitrates into ammonium sulphate by acid digestion.
(iv)WhereW0=weight of sample, gW1=weight of dried residue, gW2=weight of ash residue, gDetermination of crude protein content: The crude protein of the Chancapiedrasample was determined using the micro – Kjeldahlmethod[8]. The principle of this method is based on the transformation of protein and that of the other nitrogen containing organic compounds, other than nitrites and nitrates into ammonium sulphate by acid digestion.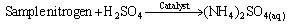 (v)
(v)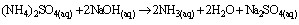 (vi)
(vi) 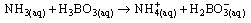 (vii)
(vii)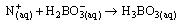 (viii)The sample (2g) was weighed along with 20cm3 of distilled water into a micro – Kjeldahl digestion flask. It was shaken and allowed to stand for sometime. One tablet of selenium catalyst was added followed by the addition of 20cm3 concentrated sulphuricacid. The flask was heated on the digestion block at 100℃ for 4 hours until the digest became clear. The flask was removed from the block and allowed to cool. The content was transferred into 50cm3 volumetric flask and diluted to the mark with water. An aliquot of the digest (10cm3) was transferred into another micro-Kjeldahl flask along with 20cm3 of distilled water, and placed in the distilling outlet of the micro – Kjeldahl distillation unit. A conical flask containing 20cm3 of boric acid indicator was placed under the condenser outlet. Sodium hydroxide solution (20cm3, 40%) was added to the content in the Kjeldahl flask by opening the funnel stopcock. The distillation start and the heat supplied were regulated to avoid sucking back. When all the available distillate was collected in 20cm3 of boric acid, the distillation was stopped. The nitrogen in the distillate was determined by titrating with 0.01M of H2SO4, the end point was obtained when the colour of the distillate changed from green to pink.Crude protein is a measure of nitrogen in the sample. It was calculated by multiplying the total nitrogen content by a constant, 6.60. This is based on the assumption that, proteins contain about 16%N which includes both true protein and non – protein N and does not make a distinction between available or unavailable protein[6]. The crude protein was calculated using equation ix% crude protein = %N x 6.60 (ix)The nitrogen content of the sample is given by the formula below.
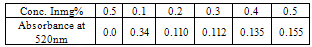
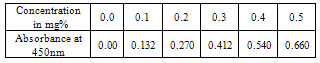
(viii)The sample (2g) was weighed along with 20cm3 of distilled water into a micro – Kjeldahl digestion flask. It was shaken and allowed to stand for sometime. One tablet of selenium catalyst was added followed by the addition of 20cm3 concentrated sulphuricacid. The flask was heated on the digestion block at 100℃ for 4 hours until the digest became clear. The flask was removed from the block and allowed to cool. The content was transferred into 50cm3 volumetric flask and diluted to the mark with water. An aliquot of the digest (10cm3) was transferred into another micro-Kjeldahl flask along with 20cm3 of distilled water, and placed in the distilling outlet of the micro – Kjeldahl distillation unit. A conical flask containing 20cm3 of boric acid indicator was placed under the condenser outlet. Sodium hydroxide solution (20cm3, 40%) was added to the content in the Kjeldahl flask by opening the funnel stopcock. The distillation start and the heat supplied were regulated to avoid sucking back. When all the available distillate was collected in 20cm3 of boric acid, the distillation was stopped. The nitrogen in the distillate was determined by titrating with 0.01M of H2SO4, the end point was obtained when the colour of the distillate changed from green to pink.Crude protein is a measure of nitrogen in the sample. It was calculated by multiplying the total nitrogen content by a constant, 6.60. This is based on the assumption that, proteins contain about 16%N which includes both true protein and non – protein N and does not make a distinction between available or unavailable protein[6]. The crude protein was calculated using equation ix% crude protein = %N x 6.60 (ix)The nitrogen content of the sample is given by the formula below.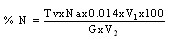 (x)Where Tv=Titre value of acid (cm3)Na=Concentration or normality of acidV1=Volume of digest and distilled water used for dilutingthe digest (50cm3).V2=Volume of aliquot used for distillation (10cm3)G=Original weight of sample used, gDetermination of carbohydrates: The James’s method[7] was adopted where the total proportion of carbohydrate in the plant sample was obtained by calculation using the percentage weight method. That is by subtracting the % sum of food nutrients: % protein, % crude lipids, % crude fiber and % ash from 100%.This is done by using the equation below.% Cx(H2O)y = 100% - (% crude protein + % crude lipid + % crude fiber + % ash) (xi)Estimation of energy value: The sample calorific value was estimated (in kcal) by multiplying the percentages of crude protein, crude lipid and carbohydrate by the recommended factors (2.44, 8.37 and 3.57 respectively) used in vegetables analysis[9]Mineral analysis: The triple acid digestion method was employed. The powder plant sample (2.0g) was weighed into a micro-Kjeldahl digestion flask to which 24cm3 of mixture of concentrated HNO3, H2SO4, and 60% HClO4 (9:2:1 v/v) were added. The flask was put on a heating block and digested to a clear solution, it was cooled and the content wastransferred into a 50cm3 volumetric flask and made-up to the volume mark with distilledwater[10]. The solution was used for determination of mineral elements; calcium, magnesium, potassium, iron, sodium, manganese, zinc and phosphorus.Minerals analysis using atomic absorption spectrometry (AAS): calcium, magnesium, potassium, iron, sodium, manganese and zinc were analyzed using atomic absorption spectrometry (AAS). The method[11] gives a good precision and accuracy. The principle of the method is based on nebulising a sample solution into an air acetylene flame where it is vaporized. Elemental ions were then atomized and the atoms then absorb radiation of a characteristic wavelength from a hallow-cathode lamp. The absorbance measured, is proportional to the amount of analyte in the sample solution. Determination of phosphorus: The clear supernatant solution (2cm3) after digestion was placed into 50cm3 volumetric flask. 2cm3 of extracting solution was added, followed by 2cm3 of ammonium molybdate solution. Then distilled water was added to make-up to 48cm3. The content was properly mixed, and 1cm3 of dilute stannous chloride solution was added and mixed again. 1cm3 of distilled water was added to make-up to 50cm3mark and left to stand for 5minutes. The percentage absorbance on thespectrophotometer at 660nm wavelength was used to determine the concentration of phosphorus[12].Determination of Oxalates content:To 1g of the powder plant sample, 75cm3 of 1.5M H2SO4 was added. The solution was carefully shaken on a mechanical shaker for 1 hour and then filtered using Whattman No.1 filter paper. The filtrate (25cm3) was then collected and titrated against 0.1M KMnO4 solution till a faint pink colourthat persisted for 30 seconds appeared. 1cm3 of 0.1M KMnO4 = 0.00450g oxalic acid[13].Determination of phytates content: The powder sample (4g) was soaked in 100cm3 of 2% HCI for 3 hours and filtered. The filtrate (25cm3), 5cm3 of 0.3% NH4SCN and 53.5cm3 of water were mixed together and titrated against standard FeCl3 solution (containing 0.00195g Fe/cm3) until a brownish yellow colour which persisted for 5 minutes appeared. Phytin – phosphorus (cm3 Fe = 1.19 mg phytinphosphorus) was determined and phytate content was calculated by multiplying the value of phytin - phosphorous by 3.55[14].Determination of tannins content: The powder sample (5g) was weighed into a 100cm3 volumetric flask. 50cm3 of distilled water was added and shaken for 1 hour on a mechanical shaker. This was filtered into a 50cm3 volumetric flask and made up to the mark with water. 5cm3 of the filtrate was pipette out into a test tube and mixed with 3cm3 of 0.1 M FeCI3 in 0.1M HCI and 3cm3 of 0.008M potassium ferrocyanide. The absorbance was measured using a spectrophotometer at 520nm wavelength, within 10min. A blank sample was prepared the colour was developed the same as the sample and absorbance read at the same wavelength. A standard was prepared using tannic acid[15]. Tannins concentration was calculated in (mg/dl) using the absorbance table and the equation below
(x)Where Tv=Titre value of acid (cm3)Na=Concentration or normality of acidV1=Volume of digest and distilled water used for dilutingthe digest (50cm3).V2=Volume of aliquot used for distillation (10cm3)G=Original weight of sample used, gDetermination of carbohydrates: The James’s method[7] was adopted where the total proportion of carbohydrate in the plant sample was obtained by calculation using the percentage weight method. That is by subtracting the % sum of food nutrients: % protein, % crude lipids, % crude fiber and % ash from 100%.This is done by using the equation below.% Cx(H2O)y = 100% - (% crude protein + % crude lipid + % crude fiber + % ash) (xi)Estimation of energy value: The sample calorific value was estimated (in kcal) by multiplying the percentages of crude protein, crude lipid and carbohydrate by the recommended factors (2.44, 8.37 and 3.57 respectively) used in vegetables analysis[9]Mineral analysis: The triple acid digestion method was employed. The powder plant sample (2.0g) was weighed into a micro-Kjeldahl digestion flask to which 24cm3 of mixture of concentrated HNO3, H2SO4, and 60% HClO4 (9:2:1 v/v) were added. The flask was put on a heating block and digested to a clear solution, it was cooled and the content wastransferred into a 50cm3 volumetric flask and made-up to the volume mark with distilledwater[10]. The solution was used for determination of mineral elements; calcium, magnesium, potassium, iron, sodium, manganese, zinc and phosphorus.Minerals analysis using atomic absorption spectrometry (AAS): calcium, magnesium, potassium, iron, sodium, manganese and zinc were analyzed using atomic absorption spectrometry (AAS). The method[11] gives a good precision and accuracy. The principle of the method is based on nebulising a sample solution into an air acetylene flame where it is vaporized. Elemental ions were then atomized and the atoms then absorb radiation of a characteristic wavelength from a hallow-cathode lamp. The absorbance measured, is proportional to the amount of analyte in the sample solution. Determination of phosphorus: The clear supernatant solution (2cm3) after digestion was placed into 50cm3 volumetric flask. 2cm3 of extracting solution was added, followed by 2cm3 of ammonium molybdate solution. Then distilled water was added to make-up to 48cm3. The content was properly mixed, and 1cm3 of dilute stannous chloride solution was added and mixed again. 1cm3 of distilled water was added to make-up to 50cm3mark and left to stand for 5minutes. The percentage absorbance on thespectrophotometer at 660nm wavelength was used to determine the concentration of phosphorus[12].Determination of Oxalates content:To 1g of the powder plant sample, 75cm3 of 1.5M H2SO4 was added. The solution was carefully shaken on a mechanical shaker for 1 hour and then filtered using Whattman No.1 filter paper. The filtrate (25cm3) was then collected and titrated against 0.1M KMnO4 solution till a faint pink colourthat persisted for 30 seconds appeared. 1cm3 of 0.1M KMnO4 = 0.00450g oxalic acid[13].Determination of phytates content: The powder sample (4g) was soaked in 100cm3 of 2% HCI for 3 hours and filtered. The filtrate (25cm3), 5cm3 of 0.3% NH4SCN and 53.5cm3 of water were mixed together and titrated against standard FeCl3 solution (containing 0.00195g Fe/cm3) until a brownish yellow colour which persisted for 5 minutes appeared. Phytin – phosphorus (cm3 Fe = 1.19 mg phytinphosphorus) was determined and phytate content was calculated by multiplying the value of phytin - phosphorous by 3.55[14].Determination of tannins content: The powder sample (5g) was weighed into a 100cm3 volumetric flask. 50cm3 of distilled water was added and shaken for 1 hour on a mechanical shaker. This was filtered into a 50cm3 volumetric flask and made up to the mark with water. 5cm3 of the filtrate was pipette out into a test tube and mixed with 3cm3 of 0.1 M FeCI3 in 0.1M HCI and 3cm3 of 0.008M potassium ferrocyanide. The absorbance was measured using a spectrophotometer at 520nm wavelength, within 10min. A blank sample was prepared the colour was developed the same as the sample and absorbance read at the same wavelength. A standard was prepared using tannic acid[15]. Tannins concentration was calculated in (mg/dl) using the absorbance table and the equation below
|
|
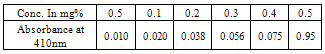
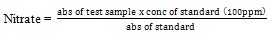 (xiii)Determination of cyanogenic glycosides: This is based on the reaction between alkaline picrate and hydrogen cyanide (HCN) resulting in an orange colour which is measured at 490nm.The lipid free sample (2.0g) was dissolved in 10cm3 of water allowed to stand for 24hours, it was then filtered and 1.0cm3 of filtrate was pipette into a test tube, 4cm3 of alkaline picrate solution was added and incubated for 5 minutes in a water bath at 90℃. The test tube was cooled to room temperature and absorbance of the solution was recorded at 490nm[8]. The concentration of cyanide in the sample was determined from the table of standards for cyanide and their absorbance below[8].
(xiii)Determination of cyanogenic glycosides: This is based on the reaction between alkaline picrate and hydrogen cyanide (HCN) resulting in an orange colour which is measured at 490nm.The lipid free sample (2.0g) was dissolved in 10cm3 of water allowed to stand for 24hours, it was then filtered and 1.0cm3 of filtrate was pipette into a test tube, 4cm3 of alkaline picrate solution was added and incubated for 5 minutes in a water bath at 90℃. The test tube was cooled to room temperature and absorbance of the solution was recorded at 490nm[8]. The concentration of cyanide in the sample was determined from the table of standards for cyanide and their absorbance below[8].
|
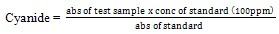 (xiv)
(xiv)3. Results and Discussion
- The results of the various analyses conducted on the sample are presented in Tables 1, 2 and 3Proximate compositionThe result revealed that the moisture content 0.03± 0.06% is lower than those of some common Nigerian leafy vegetables such as Xanthosemsagittifolum 14.7%, Vernonia amygdaline27.4% and Adansoniadigitata 9.5%[17]. The plant has low moisture content below the value of 15% above which was reported to have favour microbial activities during storage[18]. The low moisture content of the sample is an indication that they have good storage property with minimum fungal and bacterial attack [18].
|
|
|
4. Conclusions
- The Chancapiedra plant cannot provide the entire nutrient required by human system. Yet, it contains some essential nutrients like carbohydrate, iron and manganesewhich if utilized can serve as an alternative source of nutrients. It is quite safe for consumption since it contains low anti- nutritive agents such as the oxalate, phytate, cyanide and tannins contents.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML