-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(2): 16-20
doi: 10.5923/j.fph.20120202.04
Influence of Using Coconut, Palm, and Corn Oils as Frying Medium on Concentration of Acrylamide in Fried Tempe
M. Muchtaridi , J. levita , D. Rahayu , H. Rahmi
Division of Pharmacochemistry, Faculty of Pharmacy, Universitas Padjadjaran, Jl KM 21.5 Bandung-Sumedang, Jatinangor, Indonesia
Correspondence to: M. Muchtaridi , Division of Pharmacochemistry, Faculty of Pharmacy, Universitas Padjadjaran, Jl KM 21.5 Bandung-Sumedang, Jatinangor, Indonesia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
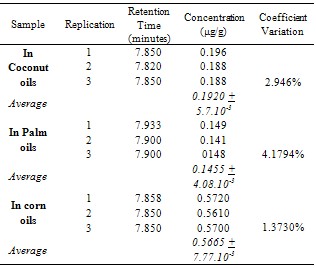
The aim of this research is to study the formation of acrylamide with different kind of vegetable oils as the cooking media. The samples were prepared by cooking and baking above 120℃ then extracted with dichloromethane-ethanol and separated by SPE (C-18) with methanol 60% as eluent. The extracts were analysed by HPLC, with condition as followed: C-18 column; acetonitrile-water (5:95) pH 2.52 mobile-phase; 0.5 ml/minute flow rate; and 210 nm wavelength. It was figured out that a fried tempe using corn oil contained 0.5778 μg/g acrylamide (8.202.10-3 standard deviation and 1.4195% coefficient variation), using coconut oil 0.192 μg/g acrylamide (5.656.10-3 standard deviation 2.946% coefficient variation), using palm oil 0.1455 μg/g acrylamide (6.081.10-3 standard deviation and 4.1794% coefficient variation).
Keywords: acrylamide, tempe, coconut oil, palm oil, corn oil
Article Outline
1. Introduction
- The Swedish National Food Authority and the University of Stockholm have conducted valuable research in the field of food safety in April 2002[1]. These researchers found microgram per kilogram to milligram per kilogram levels of acrylamide in foods[2]. Acrylamide is a reactive chemical, which is used as monomer in the synthesis of polyacrylamides used in purification of water, and in the formulation of grouting agents. Acrylamide is known as a component in tobacco smoke.The International Agency for Research on Cancer (IARC) has classified acrylamide as probably carcinogenic to humans (Group 2A). Neurological effects have been observed in humans exposed to acrylamide. Properties, use and toxic effects of acrylamide are reviewed by IARC and EU[3].Several sources of hypothesis for the formation of acrolein are known[4]. It may arise from degradation of amino acids from proteins and degradation of carbohydrates, and also from the Maillard reaction between amino acids or proteins and carbohydrates[5,6]. Glycerol is degraded to acrolein[6], the unpleasant acrid black and irritating smoke, when oil is heated at tempe fried temperatures above the smoke point (260-290°). The smoke point is higher for oils with higher content of saturated fatty acids and lower content of polyun-saturated acids. The smoke points for some of the main oils and fats are as follows: palm ℃, peanut 220℃, olive: 210℃, lard and copra 180℃, sunflower and soybean 170℃, corn 160℃, margarine 150℃, and butter 110℃[4,7-9]. Usually, the smoke starts to appear on the surface of heated oils before their tempe reaches 175℃. The oil is first hydrolyzed into glycerol and fatty acids and then acrolein is produced by the elimination of water from glycerol by a heterolytic acid- catalyzed carbonium ion mechanism followed by oxidation[2].CH2(OH)-CH(OH)- CH2(OH) à CH2=CH-CHOGlycerol,AcroleinAcrolein can be converted into acrylamide by a series of fundamental reactions. However, both acrolein and acrylamide are reactive, because of their double bonds and the amino group of acrylamide. They can readily react further with other reactive groups present in the food matrix or formed during the heating process. For example, acrylamide can react with small reactive molecules, such as urea (CO(NH2)2) and formaldehyde (HCHO), or with glyoxal ((CHO)2), aldehydes (RCHO), amines (R2NH), thiols (RSH) etc. Furthermore, the products shown in the following scheme can even react further in the same mode of reaction[2].The study of acrylamide formation has been published in many paper, even potato fried study is investigated comprehensively[10,11]. However, the study of formation acrylamide in tempe has not yet been fully investigated, for the reason that tempe are consumed almost daily in Indonesia[12]. The objective of this research would be proofed of the influence of usage of coconut oil (with 84% trygliseride), palm oil (with 84% trygliseride) and corn oil (with 98.4% trygliseride) as frying media to content of acrylamide at tempe.
2. Materials and Methods
2.1. Materials
- Chemicals. The following chemicals were obtained commercially: acrylamide pro analysis (99%, Merck), dichloromethane (Merck), ethanol and methanol grade HPLC (Merck), acetonitrile grade HPLC (Merck), phosphoric acid grade HPLC (Merck), Cartridge C-18 for SPE (Solid Phase Extraction) from Waters, Aquabidest pro injection (Ikapharmindo), KBr p.a (Merck).coconut oils, palm oils, corn oil, and tempe were obtained from a local grocery store.Instrument. HPLC: LC 10A-UV-vis SPD-10AV (Schimadzu), Vortex mixer 300, Ultrasonic shaker (NEY), Laboratory Shaker (IKA-HS 260), Spectrometry (Jena Specord 200), and pH meter.
2.2. Methods
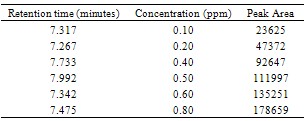
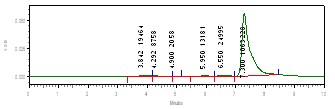
- Sample PreparationTempe was taken from Cileunyi Traditional Market in February 2006.The methods and procedure was adapted from Harahap et al.[13]. Tempe was fried until smoke point temperature by three frying media (coconut oils (sample A), palm oils (sample B), and corn oils (sample C)). The output of this process was called samples. 15 gram of samples were weighed, then it was dissolved in 60 mL dichloromethane and 3 ml ethanol ml, last be shacked with shaker laboratory at a speed at 210 rpm during 50 minute. The solution was filtered and filtrate was dissociated. The residue was cleaned by 20 mL dichloromethane, and then filtered. Into filtrate added 30 ml mobile phase, and it was evaporated in at 700℃, furthermore be packed into centrifugation tube at 8000 rpm during 30 minute. The layer of mobile phase in centrifugation was taken into volumetric flask 25 ml and added mobile phase until border. The solution in the volumetric flask was filtered with membrane filter (0.45 millipore) and 20 μL filtrate was injected into HPLC instrument. In this study, Solid Phase Extraction (C-18) was employed with methanol 60% as eluent for acrylamide extraction and clean-up[14,15].Determination of Maximum Wave Length of AcrylamideStandard solution of acrylamide was produced with dissolved 262 mg acrylamide into 250 mL of mixture solution of mobile phase (acetonitrile: H2O (5:95) pH 2.52). This standard solution was diluted to obtain concentration of 4 mg/L. solution of 4 mg/L was determined by UV with range wave length 190-390 nm.Calibration Curve10 mg/L of solution standard was diluted to obtain range concentration 2.032, 1.016, 0.813, 0.609, 0.508, 0.406, 0.203, and 0.102 mg/L. The all concentration were performed by HPLC with detector UV in 198 nm. Concentrations were plotted into area peak as shown in Fig. 1.
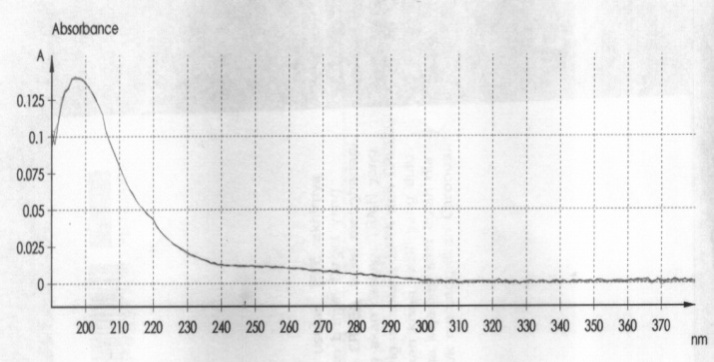 | Figure 1. Maximum Absorbance of Acrylamide at concntration 0.508 µg/ml by Using Spectrometre (UV-1700 Pharmaspec-Shimadzu) |
3. Results and Discussion
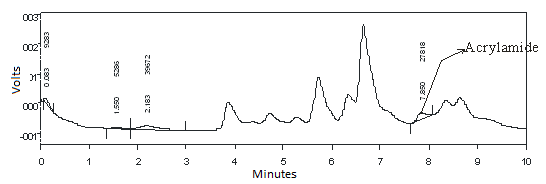
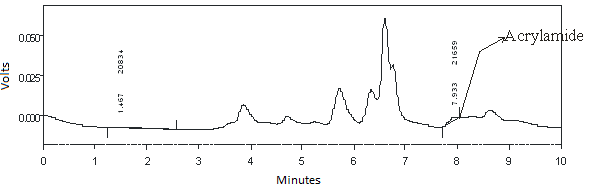
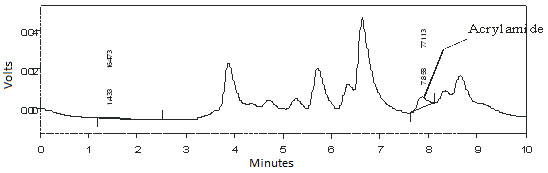
- Validation of Analysis MethodsAcrylamide had maximum absorbance on wave length 198 nm that calculated at concentration 0.508 µg/ml into dichloromethane as solvent as shown in Fig 1. This wave length was applied to evaluated sample in detector of HPLC.The various methods for determination of acrylamide includes about the occurrence, analytical methods, and extraction and clean-up procedures of acrylamide has been established, however special attention is given to chromatographic techniques applied for the occurrence and determination of acrylamide[17]. Here we studied by using HPLC with addition standard methods. In this study, combination solvents was tried to get optimum analysis. The using of acetonitrile:water (5:95) in 10% phosphoric acid gave optimum condition in analysis. As shown in Fig. 2, peak of acrylamide in chromatogram was clear at 7.30 minutes.
 | Figure 2. Chromatogram of Acrylamide use HPLC online UV detection with wave length 198 nm with mobile phase acetonitrile:water (5:95) and 10 mM phosphoric acid pH 2.52 |
|
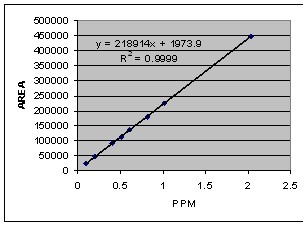 | Figure 3. Calibration curve of Acrylamide Analysis by HPLC in wave length 198 nm with mobile phase acetonitrile:water (5:95) and 10 mM phosphoric acid pH 2.52 |
|
4. Conclusions
- In this study was known that found microgram per kilogram levels of acrylamide in fried tempe by using different oils as frying medium. The average level of acrylamide in fried tempe with coconut oils, palm oils, and corn oils as frying medium were 0.5778 μg/g ( + 8.202.10-3 and coefficient variation 1.4195%) 0.192 μg/g ( + 5.656.10-3 and coefficient variation 2.946%). and 0.1455 μg/g ( + 6.081.10-3 and variation coefficient 4.1794%). respectively.
ACKNOWLEDGEMENTS
- We would gratefully acknowledge Indonesian Ministry of National education for funding this project through PHK A2- project 2008.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML