-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(2): 12-15
doi: 10.5923/j.fph.20120202.03
Determination and Residues Level of Emamectin Benzoate in Tea Using HPLC with Fluorescence Detection
Madasamy Kottiappan , Shanmugaselvan Veilumuthu Anandh
UPASI Tea Research Fondation, Tea Research Institute, Valparai, 642 127, TN, India
Correspondence to: Madasamy Kottiappan , UPASI Tea Research Fondation, Tea Research Institute, Valparai, 642 127, TN, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
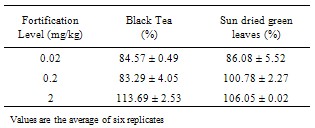
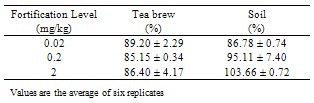
Tea is consumed throughout the world, both for pleasure and therapeutic purpose. Most people will be unaware of their involuntary exposure to residues of pesticide lingering in processed tea and so possibly transferring into infusion of tea. The objective of this trial was not only to provide a simple residue analytical method to evaluate the safe application rate of emamectin benzoate for tea crops but also suitable dosage in tea crop. The residues of emamectin benzoate were determined by high performance liquid chromatography equipped with fluorescence detector. Tea samples extracted with acetone/water (70:30 v/v), the extract were cleaned up by liquid – liquid extraction under the fortified level 0.02 µg/mg to 2.0 µg/mg, the recovery ranged from 80.90 % to 115.72 % for black tea, 82.72 % to 106.06 % for sundried green leaves, 83.85 % to 90.64 % for tea brew and 86.33 % to 104.19 % for soil. The limit of detection and limit of quantification of the method were 0.01 µg/mg and 0.02 µg/mg.
Keywords: Emamectin benzoate, Tea, Residues
Article Outline
1. Introduction
- Teas in India predominantly grow in three different areas - Darjeeling, Assam and Nilgiri. India's tea production rose by 10 % to 114.70 million kg during June, 2011, on the back of higher output in Assam and West Bengal. As an important food crop, there is an important research value on tea pesticide residues.Emamectin benzoate, a synthetic derivative of abamectin, is 4”- deoxy-4”- (epi-methylamino) avermectin B1 (MAB1) and is a mixture of two active compounds: 4”-deoxy-4”- (epi-methylamino) avermectin B1a (MAB1a) and 4”-deoxy- 4” (epi-methylamino) avermectin B1b (MAB1b). Emamectin benzoate contains the B1a and the B1b analogues in the proportion 9:1 (e.g. Figure 1). The minor structural difference between MAB1a and MAB1b coupled with the preponderance of MAB1a in emamectin benzoate and the nearly identical biological activities of the two homologues indicate that MAB1a can be used as the test substance for emamectin benzoate (e.g.[9]).Although the residue analysis of emamectin benzoate has been reported in many materials, the literatures were mostly about the multi residue determination method in soils (e.g. [1];[8]), crops (e.g.[12]), fruits and vegetables (e.g.[3]), food stuffs (e.g.[6]), and rice (e.g.[11]). Only few literatures are available with crops, such as Lettuce (e.g.[4]), Cab bage (e.g.[5]), Tomoto, Japanesh radish and Tea (e.g.[13]), rice (e.g.[14]) and Paddy (e.g.[7]). There was neither detailed simple residues analysis method nor residual dynamics report on tea.Liquid chromatographic method using a UV detector was reported for determining emamectin benzoate residues in crops (e.g.[13]). In this paper a method was developed which involves derivatization using trifluoroacetic anhydride and 1-methyl imidazole and quantification by HPLC equiped with fluorecent detector (FLD).
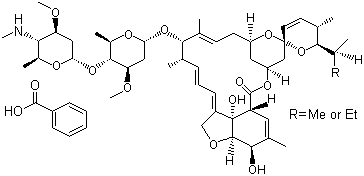 | Figure 1. Chemical structure of Emamectin benzoate |
2. Materials and Methods
2.1. Field Trials
- Field trials were carried out at two location viz., Valparai and Coonoor (Tamil Nadu, India) situated in 1050 and 1800 m above MSL respectively. The treatments were consisted of untreated control, recommended dogage and double the recommended dosage. A plot size of 700 bushes for untreated control and each treatment of the pesticide under study, leaving two rows of bushes as guard rows between the untreated control and the different treatment plots was maintained. Emamectin benzoate (Missile 5% SG) was sprayed at two dose levels, 200 g/ha (recommended dose) and 400 g/ha (double the recommended dose) with hand operated knapsack sprayer, using a recommended spray volume of 500 L/ha.
2.2. Sampling
- For studying the dissipation of emamectin benzoate in tea, samples (two leaves and a bud) were collected at time 0 day (3 h post application, when the spraying mixture had dried) and 1, 3, 5, 7, 10 and 14 days after the application. About 3 kg of the green tea shoots (two leaves and a bud) were harvested from untreated control and both treatment and brought to the laboratoy.
2.3. Tea Leaves Processing
- The untreated control and treated green tea shoots from the field were processed in the laboratory’s mini manufacturing unit, using a conventional CTC (Crush, Tear and Curl) tea manufacturing process. The manufacturing process, in brief, involved withering of shoots (50-55% water loss) at ambient temperature for 15-20 h; rolling (twisting and rupturing the tissue to experess the juice) using a rotorvane for about 15 min with pressure, followed by fermentation (oxidation for 1-2 h at 25-30℃ and 95% RH. Finally, drying in a tea dryer, using hot air at 100 ± 5℃, gave tea with final moisture content of 2-3%. The matrices used for residue determinations were the sun dried green leaves, black tea, tea brew and soil.
2.4. Chemicals
- Technical standard (96.2 % purity) and formulation of emamectin benzoate were received from M/s.Crystal Phosphate Ltd., NewDelhi. Acetone, Dichloromethane, Sodium chloride, anhydrous sodium sulphate of analytical grade and Acetonitrile of HPLC grade were purchased from M/s. Ranbaxy chemicals, NewDelhi. Florisil (60-100 mesh) purchased from M/s. Sisco Research Laboratories, Mumbai. A rotary mechanical shaker (REMI RS 36), a rotary vacuum evaporator (Labserve Super fit PBU6), millipore (Millipore Direct Q3 smart), sonicator and glass column (20 mm dia, 590 mm) were also used in this study.
2.5. Analytical Procedures
- All black tea, sun dried green leaves, tea brew and soil samples were analysed for emamectin benzoate residues by HPLC with fluorescence detector. Extraction, purification and clean-up step are briefly described below.
2.5.1. Black tea, Sun Dried Green Leaves Extraction and Clean-up Procedure
- Ten grams of black tea/sun dried green leaves sample were extracted with 200 mL (100+50+50) of acetone: water (70:30, v/v) thrice by shaking it in a mechanical shaker for 2 hours. The contents were filtered. The pooled extract was partitioned with 100 mL of dichloromethane (DCM) and collected the lower organic layer into a 500 mL round bottom flask (RBF) through anhydrous sodium sulphate. To the separating funnel containing aqueous layer, added 50 mL of saturated NaCl and 50 mL of DCM and shaken vigorously and collected the lower organic layer through anhydrous sodium sulphate into the 500 mL RBF and repeated the above steps with another 50 mL DCM. The combined DCM extract was evaporated to dryness. The concentrated residue is reconstituted an 10 mL of DCM and transferred to a glass column packed with activated florisil (10 g) with 1 cm length of anhydrous sodium sulfate above and below the florisil packing. Prior to elution, the column was washed with 50 mL of DCM to remove the co-extractives and eluted with 150 mL of 2:1 acetone and DCM mixture. The collected eluate was concentrated to dryness.
2.5.2. Tea Brews and Soil Extraction Procedure
- Weighed 2g of sample in a cleaned conical flask was adding 100 mL of boiled water and kept it over a hot water bath for 6 minutes. Filtered the brew and added 100 mL of DCM and shaken vigorously. Collected the lower organic layer through anhydrous sodium sulphate into 500 mL RBF and repeated the above steps twice with another 50 mL DCM. The pooled DCM extract was evaporated to dryness.Ten grams of soil sample were extracted with 200 mL (100+50+50) of acetone: water (70:30, v/v) thrice by shaking it in a mechanical shaker for 2 hours. The contents were filtered. The pooled extract was partitioned with 100 mL of dichloromethane (DCM) and collected the lower organic layer into a 500 mL round bottom flask (RBF) through anhydrous sodium sulphate. To the separating funnel containing aqueous layer, added 50 mL of saturated NaCl and 50 mL of DCM and shaken vigorously and collected the lower organic layer through anhydrous sodium sulphate into the 500 mL RBF and repeated the above steps with another 50 mL DCM. The combined DCM extract was evaporated to dryness.
2.5.3. Derivatization
- The emamectin benzoate residue was reconstituted in 10 mL of acetonitrile and taken 1.5 mL of this sample solution into a 5 mL standard measuring flask (SMF). Added 1 mL of 1-Methyl imidazole: Acetonitile (1:1) and 1 mL of Trifluoroacetic anhydride: Acetonitrile (1:2). Then the SMF was placed under incubation at 50℃ for 90 min. After incubation the SMF was cold in an ice bath. The mixture was made acidic by adding 100 µL acetic acid and determined emamectin benzoate residue by HPLC with fluorescence detector.
2.6. HPLC Determination
- Emamectin benzoate residues were determined with Agilent 1100 series equipped with fluorescence detector and Zorbax Rx C18 (250 mm x 4.6 mm x 5 µm) column. The injection volume was 50 µL and column oven temperature maintained at 40℃. The mobile phase consisted of acetonitrile:water (95:5 v/v) and the flow rate was 1.0 mL/min. The fluorescence detector was operated with emission and excitation wave lengths of 365 nm and 460 nm respectively.
3. Result and Discussion
3.1. Emamectin Benzoate Determination Method Efficiency
- The described method of analysis of emamectin benzoate residues in sun dried green leaves, black tea, tea brew and soil by HPLC is fast and relatively simple. Quantification was accomplished by using a standard curve prepared by diluting the stock solution in acetonitrile. A good linearity was achived with a correlation coefficient of 0.9957. The limit of detection (LOD) was determined, based on the lowest level of standard concentration detected and was found to be 0.01 µg/mg. All untreated control samples show no evidence of chromatographic interference in samples of sun dried green leaves, black tea, tea brew and soil. Confirmation of emamectin benzoate in samples was performed by assessing its retention time.The efficiency of the method has been evaluated by spiking sun dried green leaves, black tea, tea brew and soil samples with emamectin benzoate working solutions at various levels (0.02-2.0 µg/mL). Recovery values for black tea, sundried green leaves, tea brew and soil are reported (e.g. Table 1 and 2). All these values of recovery indicated good method accuracy and repeatability, and are within the acceptable range for residue determination.
|
|
3.2. Dissipation of Residues
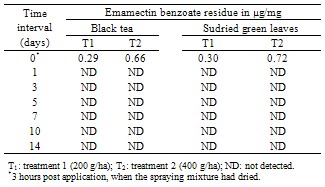
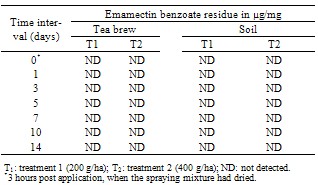
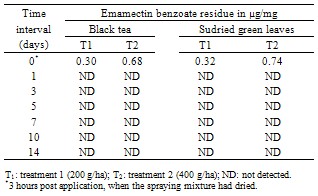
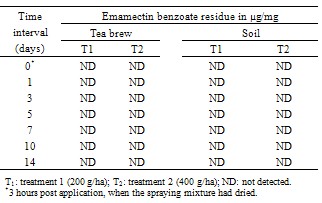
- The data relating to the residues in different matrices of tea from the field experiments carried out in September, 2011 (wet season) are reported in Table 3 and 5. No residues of emamectin benzoate were detected in any analysed untreated control tea samples. The initial deposits of the emamectin benzoate residues in black tea at the two different dosages were 0.29 and 0.66 µg/mg in valparai and 0.30 and 0.68 µg/mg in coonoor. Similarly, In the case of sun dried green leaves, residues observed on day 0 were 0.30 and 0.0.72 µg/mg in valparai and 0.0.32 and 0.74µg/mg in coonoor. It is evident from the data that emamectin benzoate degraded more rapidly and after 0 day no residues were found in black tea and sun dried green leaves at both dosages. In the tea field, besides the effect of some physical and chemical factors, like light, heat, pH and moisture (e.g. [10]) might have played a significant role in the degradation of emamectin benzoate residues.There was no detectable residue in soil at both dosages reported in Table 4 and 6.
|
|
|
|
4. Conclusions
- The described method of analysis of emamectin benzoate residues is suitable for determination of residues in tea and the method can be suitably applied to the other crops. It is suggested that the data on leaching of residues into tea brew should be taken into account for fixing a realistic MRL for pesticides in black tea.
ACKNOWLEDGEMENTS
- We acknowledged the support and grants from UPASI Tea Research Institute, Valparai and M/s. Crystal Phosphate Ltd., NewDelhi.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML