-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(1): 16-23
doi: 10.5923/j.fph.20120201.04
Assessment of Total Lipid Fractions and Fatty Acids Composition in Raw, Germinated Barleys and Talbina Products
M. Kamal E. Youssef , Fawzy Abd El-Kader El-Fishawy , El-Sayed Abd El-Naby Ramadan , Asmaa MohamedAbd El-Rahman
Food Sci & Tech. Dept, Faculty of Agric. Assiut Uni, Assiut. Egypt
Correspondence to: Asmaa MohamedAbd El-Rahman , Food Sci & Tech. Dept, Faculty of Agric. Assiut Uni, Assiut. Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Talbina is a food product with high potential applications as a functional food. Talbina was prepared from two barley varieties namely: Giza126 and Giza130 by adding whole barley flour to water (1:10 w/v) and (1:5 w/v) for germinated barley then heating at 80° C for 5 minutes with continuous stirring until reaching a porridge like texture. The study included the fractionation and determinations of the compounds of raw, germinated barley, talbina, germinated talbina and commercial talbina oil. Besides, the all treatments recorded rather slight decrease in crude fat content, which ranged from 1.5 to 2.9%. Using TLC technique the total lipids of raw, germinated barley and their talbina products were fractionated to eight fractions and triglycerides showed the highest percentage among lipid fractions (33.92-60.82%) followed by 1,3diglycerides (6.78-20.43%). The fatty acids analysis revealed that there were 11 fatty acids in the studied treatments oil namely: caprylic, lauric, myristic, palmitic, stearic, arachidic, oleic, linoleic, linolenic and gadoleic. The essential fatty acid linoleic (C18:2) recorded the highest percentage of the unsaturated fatty acids (53.59%) in germinated 130. While palmitic acid (C16:0) recorded the highest value of saturated fatty acids in germinated 126 (17.44%).
Keywords: Barley, Talbina, Germination, Functional Properties, Total Lipids, Fatty Acids
Cite this paper: M. Kamal E. Youssef , Fawzy Abd El-Kader El-Fishawy , El-Sayed Abd El-Naby Ramadan , Asmaa MohamedAbd El-Rahman , "Assessment of Total Lipid Fractions and Fatty Acids Composition in Raw, Germinated Barleys and Talbina Products", Food and Public Health, Vol. 2 No. 1, 2012, pp. 16-23. doi: 10.5923/j.fph.20120201.04.
Article Outline
1. Introduction
- Barley (Hordeum Vulgare.L) is an ancient and important cereal grain and occupies about 9.4% of the total world area under cereal production and ranks fifth in the world (Sharma and Gujral, 2010). Barley is available in both hulled and hull-less forms, hull-less barley, which does not require dehulling,offers some advantages for food uses; hulless barley had been shown to have greater nutritional value compared to covered barley in several studies with monogastric animals (Bhatty et al., 1979).Water absorption capacity (WAC) measure the volume occupied by the granule or starch polymer after swelling in excess water, while the water solubility index (WSI) determines the amount of free polysaccharide or polysaccharide released from the granule after addition of excess water (Sriburi &Hill, 2000). Oil absorption is very important as it affects textural and sensory properties of food through enhancement of flavor retention and improvement of mouth feel, key function of fat (Hung and Zayas, 1992)Lipids are located throughout the barley kernel and are classified into two basic fractions: nonstarch lipids and starch lipids. Nonstarch lipids include all lipids other than those inside starch granules. Lipids are also classified as polar or non polar, a characteristic that affects solubility and depends on molecular structure (Rosemary et al., 2008). Lipid is concentrated in the embryo, and although the embryo represents only about 3% of the kernel by weight, it furnishes about 18% of the total lipid in barley, while the endosperm contains about 3% lipid, but by virtue of size, furnishes about 77% of the total lipid, Most of the endosperm lipid is found in the aleurone layer. The remaining 5% of the total kernel lipid is found in the hull (Price and Parsons 1975, 1979; Briggs 1978). Furthermore, Erkan et al., (2006) reported that fat ranged between 1.62-1.92% in hulled barley and 1.9% in hull-less barley. Similar results were shown by Welch (1978), who found that oil content ranged from 1.9 to 4.1% and represented positive correlation with protein content. Price and Parsons, (1979) studied the composition of fatty acids from hulless barley and their research revealed that linoleic acid was the predominant unsaturated fatty acid and palmitic acid was the major saturated fatty acid of the neutral lipids, phospholipids and glycolipids, which is in agreement with our results.Morrison (1993a) also noted that the fatty acids in barley are similar to those in wheat except that barley tends to have more Linolenic acid than wheat.; also he was reported that palmitic acid was the predominant acid in the free fatty acids, followed by linoleic and oleic acids in that order. The relatively high linoleic acid content makes the oil nutritionally valuable for its effects on cardiovascular disease and cancer prevention (Wahle et al, 2004). Moreover, Qian, (2009) determined the fatty acids in the hulless barley bran oil, and they found that characterized by linoleic and palmitic acids as the major fatty acids. Talbina is an Arabic word made of the word laban which means milk, this may also designate in the case of barley grains when they reach the milky stage, so the inside of these grains is white and liquid resembling milk (Abd El-Hassib, 2007).The present study was carried out in an attempt to clarify the lipids content as well as the fatty acid composition were determined in two Egyptian raw barley varieties (Giza 126 & Giza 130) and their germinated forms as well as the talbina which made from both, in an attempt to assess their nutritive value.
2. Materials and Methods
2.1. Materials
- Ten kilograms of each varieties of Egyptian barley grains (Hordeum Vulgare L.): Giza126 (hulled barley), and Giza130 (hull-less barley) were procured from Agricultural Research Center, Giza (ARE). 100g Commercial talbina (Giza132) was obtained from local market in Assiut Governorate. All samples were obtained in year 2008.
2.2. Methods
2.2.1. Preparation of Samples
2.2.1.1. Preparation of Germinated Barley
- Soaking: Seeds were freed from broken seeds, dust and other foreign materials, and then it soaked in water (1:5 w/v) for 12 h at 25±5℃. Germination: The presoaked (12 h) seeds were spread on wet cotton in aluminum baskets. The temperature ranged from 10℃ (during the first 144 h) to 25±5℃ (during the last 24 h of sprouting). The germinated seeds were dried at 55℃ for 24 h then at 71℃ for the same period.
2.2.1.2. Preparation of Talbina
- Talbina was prepared by adding whole barley flour to water (1:10 w/v) according to Youssef (2008) and (1:5 w/v) for germinated barley flour then the mix was heated at 80 ± 5℃ for 5 minutes with continuous stirring until reaching a porridge like texture as described in Fig. (1).
2.2.2. Analytical Methods
2.2.2.1. Determination of water absorption capacity, water solubility index and oil absorption capacity of flour:
- Water absorption capacity of flour was measured by the centrifugation method of (Sosulski, 1962). Flour (3 g) was dispersed in 25 ml of distilled water and placed in pre-weighed centrifuge tubes. The dispersion was stirrer for 10 min followed by centrifugation for 25 min at 3000×g. The supernatant was drained off by allowing the tube to stand inverted for 10 min. The supernatant obtained from water absorption capacity were collected in pre weighed and dry Petri plates and dried in hot air oven for 24 h at 105℃ temperature. The results were expressed as % water solubility index. The oil absorption capacity was determined according to method of Lin et al., (1974). Flour (0.5 g) was mixed with 10 ml of refined oil in pre-weighed centrifugal tube and vortexes for 10 min. The tubes were centrifuged for 25 min at 3000×g. The oil was drained off by inverting for 10 min and centrifuge tubes were weighed.
2.2.2.2. Lipids analysis
2.2.2.2.1. Determination of crude oil
- Determination of total Lipids content:Total lipids content was determined according to the method described in the A.O.A.C. (2000) by the standard soxhelt extraction method using petroleum ether extraction.
2.2.2.2.2. Lipids Extraction
- Lipids were extracted from the dried samples of raw barleys, germinated barleys, talbina, germinated talbina and commercial talbina by chloroform: methanol mixture (2:1, v/v) according to the method described by (Folch et al., 1957).
2.2.2.2.3. Thin layer Chromatographic for Total Lipids
- The total lipids of raw barleys, germinated barleys, talbina, germinated talbina and commercial talbina were fractionated by the method of Malins and Mangold (1960) using silica gel (Gf 254, type 60) glass plates (20 ×20 cm) with 0.25 mm thickness were used for qualitative and quantitative determinations of lipid fractions. The developing solvent system was petroleum ether: diethyl ether and glacial acetic acid (80:20:1 v/v/v) (Kates, 1972).The lipid fractions were visualized by exposure to iodine vapor. All lipid fractions were identified on thin layer plates by comparing their Rf values with those of known lipid standers. For quantitative analysis, the different lipid fractions were scanned by using densitometer model (Seroscan elvi 146) and the data were analyzed by QS computer program analysis J scans. The percentage of each component was calculated with regard to the total area as follows:
2.2.2.2.4. Preparation of Methyl Esters of Fatty Acids
- The methyl esters of fatty acids were prepared from aliquots of total lipids using 5 ml 3% H2SO4 in absolute methanol and 2 ml benzene as described by (Rossell et al., 1983).
2.2.2.2.5. GLC- of Methyl Ester of Fatty Acids
- The methyl esters of fatty acids were separated using a PYE Unicam Pro-GC gas liquid chromatography with a dual flame ionization carried out on (1.5m×4 mm) SP-2310 column, packed with 55% cyanopropyl phenyl silicone dimensions. Column temperature: at first increasing the temperature from 70 to 190℃ at the rate of 8℃/ minute and then isothermal for 10 minute at 190℃. The injector and detector temperature were 250℃ and 300℃; respectively. Carrier gas: Nitrogen at the rate 30 ml/ minute, hydrogen and air flow rate were 33 and 330ml/ minute; respectively. The chart speed was 0.4 cm/ minute. Peak identifications were established by comparing the retention times obtained with standard methyl esters. The areas under chromatographic peak were measured with electronic integrator as mentioned by (Rossell et al., 1983).
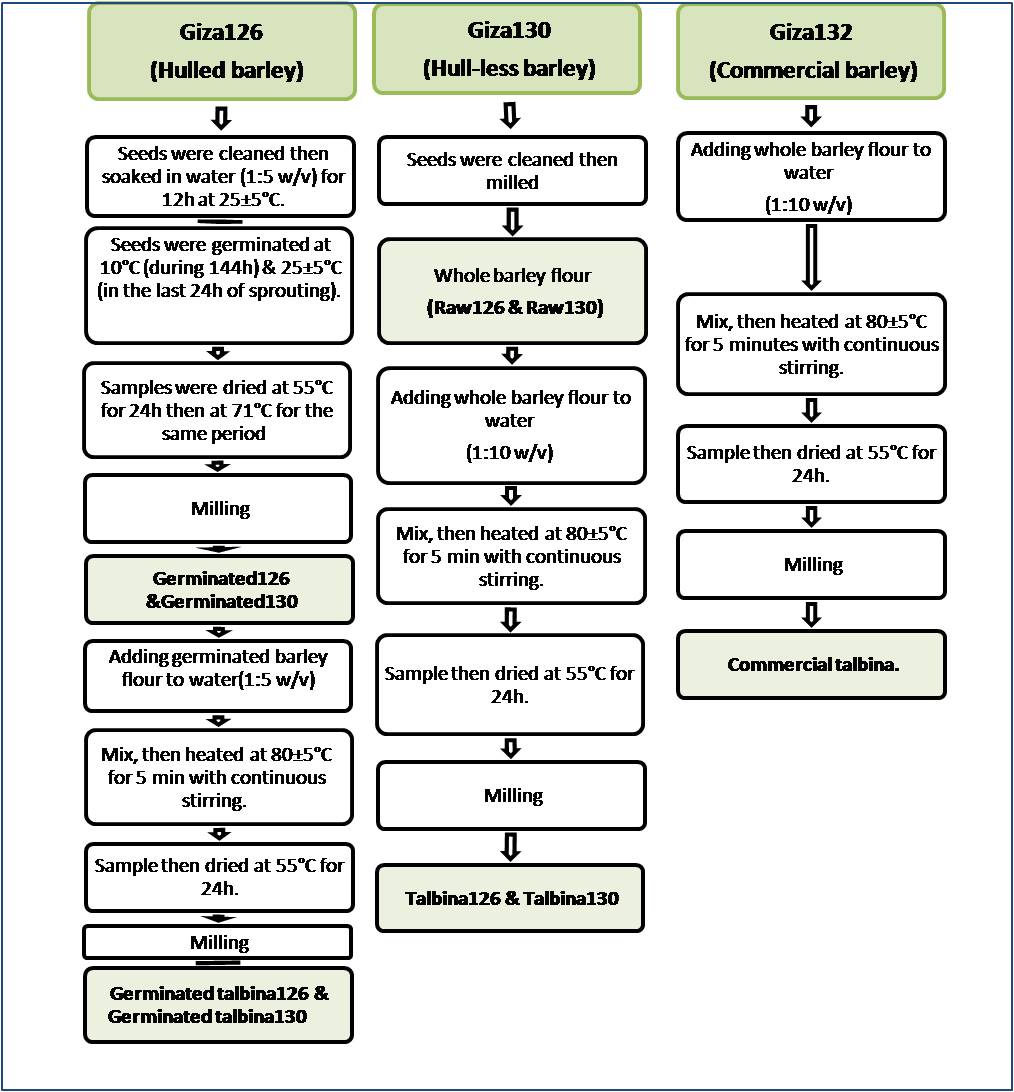 | Figure 1. Flow sheet for the preparation of talbina products |
3. Results and Discussion
3.1. Functional Properties
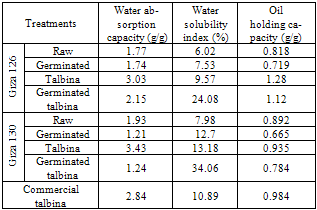
- The data revealed in Table (1) showed that (WAC) was decrease after germination treatment in both varieties, and that decrease might be due to degradation of starch in sprouting seeds. In this concept, Fincher and Stone (1986) reported that, during the germination of barley grain the nutritional reserves of the endosperm, mainly starch and proteins, had to be mobilized by a set of hydrolytic enzymes synthesized in the aleurone layer and in the scultellum of the germinating grain. Moreover, Greenwood and Thamson (1959) found that the percentage of starch in the malted barley kernels was less than that in the original barley. Kinsella (1976) reported that, the function of proteins could hold water by hydrogen bonding or by physical entrapment. On the other hand, (WAC) was increase after talbina treatment, and that increase might be due to ß-glucans which form viscous gels on hydration. Likewise, Munck (1981) found nonstarch polysaccharide ß-glucan may influence (WAC). In this concept, Burkus and Temelli (2005) mentioned that for accurate rheological measurements of ß-glucan gum, that would be complete hydration of the gum tested, e.g ≥30 min at 80-85°C for ß-glucan is absolutely essential. Moreover, Diwakar et al., (1996) mentioned that during cooking, gelatinization of the carbohydrates and swelling of the crude fiber might occur which could also lead to increase water absorption. On the contrary, Pelemba et al, (2002) found a decrease in (WAC) with increasing temperature and they explained that by decomposition or degradation of starch. Ding et al, (2006) also stated that the WAC decreased with increasing temperature if starch melting prevails over the gelatinization phenomenon. Water absorption capacity in eight hulled barley cultivars ranged from 1.38 to 1.63 g/g, where oil holding capacity significantly varied among the cultivars and ranged from 1.5 to 1.68 g/g (Sharma and Gujral, 2010). Whereas, in the present study (WAC) was 1.77& 1.93, 1.74 & 1.21, 3.03 & 3.43, 2.15 & 1.24 and 2.84 in raw, germinated barleys, talbina, germinated talbina (Giza 126 & Giza 130) and commercial talbina (Giza 132); respectively. Bhatty (1993) reported similar value for water holding capacity for barley flour.Water solubility index (WSI) increase after all studied treatments, and it ranged from 6.02% to 34.06%, that increase due to hydrolysis for polysaccharides during malting. Likewise, the hydro-thermal treatment for barley flour (talbina) had an increase in (WSI), which happened as a result of increase the ratio of ß-glucan solubility, which indicated in table (1). It could be seen from Table (1) that Oil holding capacity (OAC) ranged from 0.719-1.28 g/g. In this concept, Sosulski and Cadden (1982) found that fiber sources rich in lignin showed high (OAC). Such a relationship between fiber and (OAC) appeared in talbina 126, which had the highest value of (OAC), and highest value of insoluble dietary fiber. Furthermore, the protein denatured during cooking and the non polar residues from the interior of the protein molecule could unmask the fat which ultimately led to increase in oil absorption (Pawar and Ingle, 1988). Fig (2&3) showed the ratio of the change in functional properties by the all studied treatments.
|
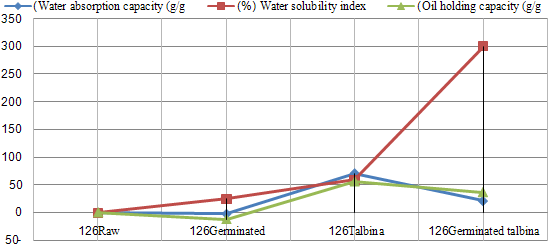 | Figure 2. The effect of germination and thermal treatment on functional properties (Giza126) |
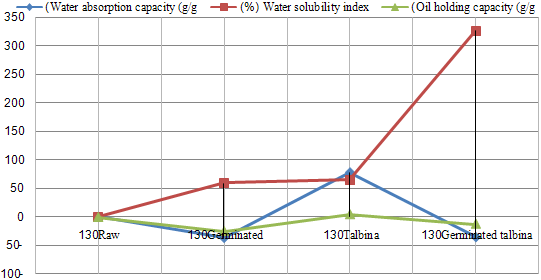 | Figure 3. The effect of germination and thermal treatment on functional properties (Giza130) |
3.2. Lipids Composition
3.2.1. Total lipid Fractions
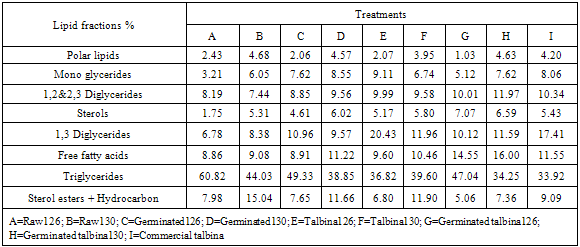
- Chromatograms of the several of lipid fractions isolated from raw barleys, germinated barleys, talbina, germinated talbina and commercial talbina are shown in Fig. (4) & (5). These fractions are identified as follows: polar lipids; monoglycerides; 1,2&2,3 diglycerides; Sterols; 1,3 diglycerides; Free fatty acids; Triglycerides and Sterol esters + Hydrocarbons.
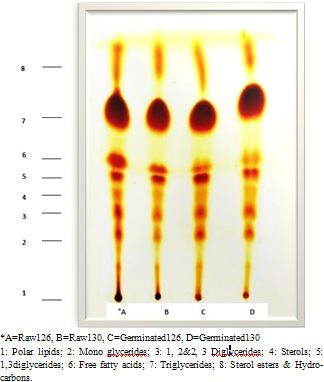 | Figure 4. Thin layer chromatograms of total lipids fractions in raw and germinated barley |
 | Figure 5. Thin layer chromatograms of total lipids fractions in talbina products |
3.2.2. Fatty Acid Composition
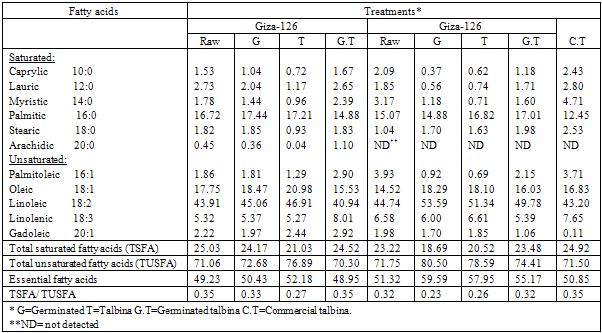
- Results of gas chromatographic analysis of the methyl esters of saturated and unsaturated fatty acids of raw, germinated barley and talbina products are presented in Table (3). The data indicated that unsaturated fatty acids represented 71.06 &71.75, 72.68 &80.50, 76.89 &78.59, 70.30 &74.41 and 71.5%. Whereas, saturated fatty acids recorded lower value, which were 25.03& 23.22, 24.17 &18.69, 21.03 & 20.52, 24.52 & 23.48 and 24.92% of raw, germinated barleys, talbina, germinated talbina and commercial talbina; respectively. Such data showed that, both of germination and talbina treatments had an increment in unsaturated fatty acids and recorded the lowest value of saturated fatty acids. Furthermore Salem, (1997) determined the fatty acids methyl ester chromatogram of lipid extracted from Giza 123 barley variety and she found their percentage in a decreasing order were: linoleic acid 46.39%, palmitic acid 21.17%, oleic acid 20.64% followed by linoleic acid 6.42% and stearic acid 2.12%, myrisitic acid 0.39%, caprylic acid 0.119% and lauric acid 0.031%.Linoleic and alpha-Linolenic acids, as the two representative compounds of n-6 and n-3 fatty acid, are essential fatty acids in mammalian nutrition (Benatti et al., 2004). The recommended ratio of n-6 to n-3 fatty acids is estimated to be from 3 to 5 (Schaefer., 2002), so the all studied samples were suitable for being used as an edible oil directly, and their oil can be used as a good source of linoleic acid. The relatively high linoleic acid content makes the oil nutritionally valuable for its effects on cardiovascular disease and cancer prevention (Wahle et al., 2004). Moreover, the essential fatty acid linoleic (C18:2) recorded the highest percentage of the unsaturated fatty acids (53.59%) in germinated 130. While palmitic acid (C16:0) recorded the highest value of saturated fatty acids in germinated 126 (17.44%). These results are in agreement with Rosemary et al, (2008); Qian et al, (2009) and Weihrauch et al, (1976). Besides, it could be seen from Table (3) that, germination caused an increase in stearic, oleic and linoleic, while, capric, lauric, myristic, Palmitoleic and eicosenoic were decrease. Zimmerman and Klosterman (1965) mentioned that, changes in the fatty acids after germination might be due to the lipolytic activity of lipases. On the other hand cooking processes (talbina) recorded an increment in palmitic, oleic and linoleic.The saturated/ unsaturated ratios were 00.352 & 0.323, 0.333 & 0.232, 0.274& 0.261, 0.348 & 0.315 and 0.348 in raw, germinated barley, talbina, germinated talbina and commercial talbina; respectively. Likewise, Salem (1997) reported that saturated/ unsaturated ratio in lipid extracted from Giza 123 barley variety was 0.317.
|
|
4. Conclusions
- Based on our study, germinated barley grain and talbina considered a resource for the pharmaceutical industries and for edible purposes, such as the production of conjugated linoleic acid and food emulsifiers, and its applications in these fields also needs further research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML