-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2012; 2(1): 11-15
doi: 10.5923/j.fph.20120201.03
Effect of Processing and Cooking Methods on the Chemical Composition, Sugars and Phytic Acid of Soybeans
Ramadan E. A.
Department of Food Science and Technology, Faculty of Agriculture, University of Assiut Assiut, Egypt
Correspondence to: Ramadan E. A. , Department of Food Science and Technology, Faculty of Agriculture, University of Assiut Assiut, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
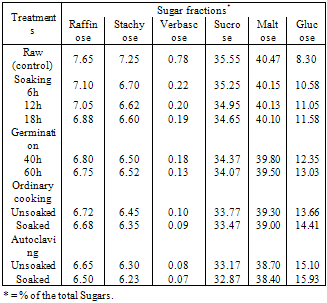
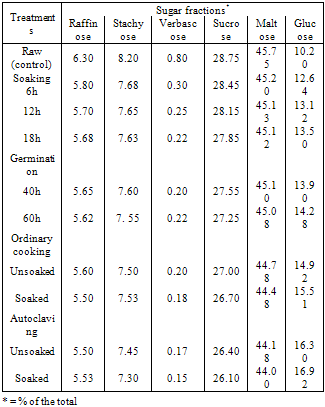
The present investigation was conducted to study the effect of processing (soaking and germination) and cooking methods (ordinary cooking and autoclaving) on the chemical composition, sugars and phytic of two varieties soybean seeds, Giza, 21 and Giza, 35. The processing and cooking methods caused increase in both protein and crude fiber contents. Meanwhile, crude oil and carbohydrates contents were decreased of the studied soybean seeds. Generally, the processing and cooking methods resulted in a decrease of raffinose, stachyose, verbascose, maltose and sucrose accompanied by an increase in glucose. These resulted revealed that the processing (soaking and germination) and cooking methods (ordinary cooking and autoclaving) was more effective in eliminating the contents of oligosaccharides and phytic acid in both varieties soybean seeds.
Keywords: Soybeans, Phytic Acid, Sugars, Soaking, Germination, Autoclaving
Cite this paper: Ramadan E. A. , "Effect of Processing and Cooking Methods on the Chemical Composition, Sugars and Phytic Acid of Soybeans", Food and Public Health, Vol. 2 No. 1, 2012, pp. 11-15. doi: 10.5923/j.fph.20120201.03.
1. Introduction
- Grain legumes are the major sources of dietary proteins in all the developing countries because animal proteins are expensive. Information on the amounts of oligosaccharides extracted from soybean seeds during cooking has been reported by Ku et al. (1976). The traditional method for home preparation of this leguminous seed consists of a water soaking period (usually overnight) followed by cooking of the dehydrated seeds after discarding the soaking water. Beans become edible after prolonged boiling in water or after a shorter time when a pressure cooker is used (Silva and Braga, 1982).The oligosaccharides, raffinose, stachyose and verbascose, common in legume seeds, are thought be the major producers of flatulence. These saccharides are comprised of one, two and three glactose units respectively joined together with sucrose in α-D linkages. Owing to the lack of α-galactosidases in mammalian digestive system, they pass into the colon where they may produce diarrhea, flatus gas (Co2, H2 and small amounts of CH4 gases) and their inevitable social discomfort (Wagner et al. 1976, Fleming, 1981 and Vijayakumari et al. 1996).Domestic processing and cooking methods are known to reduce the antinutrients and thus improve the nutritive value of legume grains (Khokhar and Chauhan, 1986 and Sharma and Sehgal, 1992). Germination caused the most significantreduction in phytates. The longer period of germination, led to greater reduction in phytic acid content; germination of seeds for 48 h caused a reduction of 66 to 69%. This reduction was possibly due to activation of phytase during germination (Eskin and Wiebe, 1983 and Sharma and Sehgal, 1992).Phytic acid, myo – inositol 1, 2, 3, 4, 5, 6– hexa – kis (dihydrogen phosphate) widely distributed in mature legume grains, stores most of the grain phosphorus. Phytic acid has antinutritional properties owing to its ability to chelate several minerals and thereby reduce their bioavaibility (Nolan and Duffin, 1987, and Vijayakumari et al. 1996). The effect of germination conditions on the chemical composition, biochemical constituents and antinutritional factors of soybean was studied (Bau et al. 1997).Therefore, the present study was undertaken to investigate the effect of various domestic processing (soaking and germination) and cooking methods (ordinary cooking and autoclaving) on the chemical composition, sugars and phytic acid of two varieties of soybeans.
2. Materials and Methods
- Soybean Giza, 21 and Giza 35 seeds were obtained from the Food Legume Section, Field Crops Research Institute, Agriculture Research Center, Giza, Egypt during 2008/2009. Grains were cleaned and other foreign materials were discarded. The cleaned seeds were stored in polyethylene bags under refrigeration until used.Chemical CompositionMoisture, crude protein, crude oil, crude fiber and ash contents were determined as described by AOAC (1990). Total carbohydrates were calculated by difference.Extraction and determination of sugars:Sugars were extracted according to the method of Vijayakumari et al. (1996). 5g of each sample treating with 25ml of 80% ethanol at room temperature by repeated shaking. The extraction was repeated twice. The extracts were pooled and concentrated under vacuum at 4°C. The residue was made up to 5ml with distilled water, and the sugars were separated using ascending thin layer chromatography and the solvent mixture, n-butanol- ethyl acetate- isopropanol - acetic acid - water (35: 100: 60: 35: 30) according to Lato et al.(1968). After development, the plate was visualized by spraying α-naphthol reagent. 10.5 ml α-naphthol (15% solution in 95% ethanol w/v), 6.5 ml concentrated sulfuric acid, 40.5ml 95% ethanol and 40 ml water (Jacin and Mishkin 1965). The isolated sugars were identified according to the Rf values of standard sugars on the same plate. The quantitative analysis of separated chromatographic fractions of sugar was estimated by scanning using Shiuadzu TLC- Scanner (C – S – 910).Extraction and estimation of phytic acidPhytic acid content was determined as described by Wheeler and Ferrel (1971). Phytic acid was extracted from 3g seed flour with 50ml of 3% TCA by shaking at room temperature followed by high speed centrifugation. The phytic acid in supernatant was precipitated as ferric phytate by adding excess ferric chloride and centrifuged. The ferric phytate was converted to ferric hydroxide with a few ml of water and 3ml of 1.5N NaOH, and then the iron content present in the sample was estimated. The phytate phosphorus was calculated from the iron results assuming a 4 : 6 iron : phosphorus molecular ratio. The phytic acid was estimated by multiplying the amount of phytate phosphorus by the factor 3.55 based on the empirical formula C6 P6 O24 H18.Processing and cooking methodsSoakingSeeds were soaked in tap water, (seeds : water, 1 : 5 w/v) for 6, 12 and 18h at room temperature (30°C). The water left after soaking was discarded. The soaked seeds were washed twice with ordinary water followed by rinsing with distilled water and then dried in a hot air oven at 60°C. (Kaur and Kapoor, 1990). Dried samples were ground to (60 mesh sieve) and then stored in air – tight plastic containers for further chemical analysis.GerminationThe seeds were placed in sterile Petri dishes lined with wet filter paper and kept in an incubator at 30°C for 40 and 60h with frequent watering. The sprouted samples were dried in a hot air oven maintained at 60°C, ground (60 mesh sieve) and stored in glass bottles under refrigeration for further analysis (Kaur and Kapoor, 1990).Ordinary cookingThe soaked seeds (12h in tap water) and unsoaked seeds were cooked in beakers. The ratio of seeds to water was 1: 5 and 1: 6 (w/v) for soaked and unsoaked seed, respectively. The water was allowed to boil before the addition of seeds. The seeds were cooked until soft as felt between fingers. The cooking time was 10 and 15 min for soaked and unsoaked seeds, respectively. The cooked samples were then mashed and dried in a hot air oven maintained at 60°C and then ground to a fine powder (60 mesh sieve) and stored (Kaur and Kapoor, 1990).AutoclavingThe soaked seeds for 12h and unsoaked seeds were autoclaved at 1.05 kg/cm2 for 5 and 10 min, respectively. The ratio of seed to water was 1: 5 (w/v) for unsoaked seeds and 1: 4 (w/v) for soaked seeds. The autoclaved seeds were then mashed, dried at 60°C finely ground (60 mesh sieve) and stored. (Kaur and Kapoor, 1990).
3. Results and Discussion
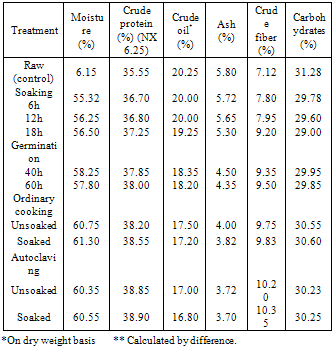
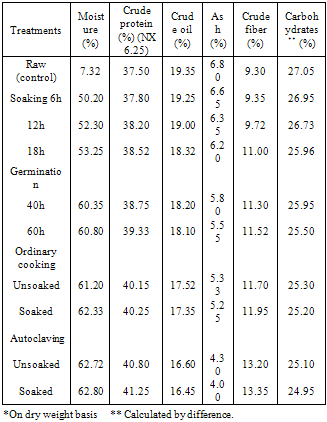
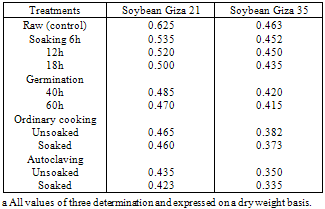
- The effect of processing and cooking methods on chemical compositionThe gross chemical composition of soybean; Giza 21 and Giza 35 of processing and cooking methods are shown in Tables (1 and 2). The data showed that, the protein content of soybean Giza 21 (35.55%) and Giza 35 (37.50%).Processing (soaking and germination) and cooking methods (ordinary cooking and autoclaving) caused a slight increase in protein content of tow varieties soybean Giza 21 and Giza 35. Similar findings were reported by Akinlosotu and Akinyele (1991). Lee and karunanithy (1990) observed also an increase in total crude protein content of more than 21% in dehulling germinated soybean compared to ungerminated seeds. Awad – Allah (1996) observed that the protein content was (37.61%) in soybean seed variety "Calland" which was characterized by relatively less lipid content (19.16%). Opposite phenomena was show in the soybean seed variety "Williams" which were 33.83% and 21.04%, respectively.The crude oil content of soybean; Giza 21 (20.25% and Giza 35 (19.35%) are shown in Tables (1 and 2). The crude oil content was slightly decreased after processing (soaking and germination) and cooking methods (ordinary cooking and autoclaving) of soybean seed, Giza 21 and Giza 35. This decrement in oil content might be due to the increasing activity of lipases during soaking and germination (kylen and MeCready, 1975), as well as the breakdown of oil, into glycerol and fatty acids (Igbedioh et al. 1994). The lipid content of soybean seeds gradually diminishes as germination progresses. At the end of 5 days, loss of lipids reached 19.8% compared to ungerminated seeds (Chandrasiri et al. 1990). Mostafa et al. (1987) found also that 6 – day's germination induced a decrease in oil content of soybean seeds.The crude fiber contents of soybean; Giza 21 and Giza 35 recorded an increase during processing (soaking and germination) and cooking methods (ordinary cooking and autoclaving), Tables (1 and 2). Awad- Allah (1996) found that the crude fiber of the soybean seed varieties "Williams and Calland" were 7.11% and 7.43%, respectively.
|
|
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML