-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2024; 14(1): 23-31
doi:10.5923/j.food.20241401.02
Received: Oct. 6, 2022; Accepted: Nov. 10, 2022; Published: May 31, 2024

Colour and Carotenoids Profiles of Fresh and Dried Pepper (Capsicum annuum var abbreviatum and Capsicum annuum var grossum)
Alamu A. E.1, Akinwande B. A.2, Ade-Omowaye B. I. O.2, Dudu O. E.1, Odedoyin R. A.1
1Department of Food Science and Technology Bells University of Technology, Ota, Ogun State, Nigeria
2Department of Food Science Ladoke Akintola University of Technology, Ogbomoso, Oyo state, Nigeria
Correspondence to: Alamu A. E., Department of Food Science and Technology Bells University of Technology, Ota, Ogun State, Nigeria.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, fresh Capsicum annuum var abbreviatum (AA) and Capsicum annuum var grossum (AG) grown in selected locations of Oyo State in Nigeria was subjected to sun (SAA, SAG) and cabinet (CAA, CAG) drying methods. Moisture content, water activity, carotenoid profiles, extractable and surface colors of the peppers were determined. The moisture content significantly reduced (p<0.05) with cabinet drying as 88.39 and 88.24% loss were recorded in AA and AG samples. Capsorubin (42.9 and 15.86%) and capsanthin (10.4 and 8.98%) loss were reported in AA and AG due to sun drying while capsorubin (29.1 and 41.9% and capsanthin (6.6 and 0.57%) loss due to cabinet drying respectively. Antheraxanthin and zeaxanthin was not detected in sun-dried samples. Extractable colour losses of 41.63 and 30.58% in SAA and SAG were significantly higher compared to 25.71% and 23.08% recorded for CAA and CAG. The chromatic coordinates redness, yellowness and lightness values (a*, b* and L*) of AA and AG decreased with the drying methods with values ranging from 24.61- 41.35, 20.24-35.51 and 36.41-52.37, respectively. Drying significantly affected the browning index and the colour difference values with the cabinet drying method showing higher values with sampleAG. Thus, cabinet drying may be considered a better alternative to sun drying for dehydration of red pepper.
Keywords: Pepper, Drying, Carotenoids, HPLC, Colour properties, Browning Index
Cite this paper: Alamu A. E., Akinwande B. A., Ade-Omowaye B. I. O., Dudu O. E., Odedoyin R. A., Colour and Carotenoids Profiles of Fresh and Dried Pepper (Capsicum annuum var abbreviatum and Capsicum annuum var grossum), International Journal of Food Science and Nutrition Engineering, Vol. 14 No. 1, 2024, pp. 23-31. doi: 10.5923/j.food.20241401.02.
Article Outline
1. Introduction
- Pepper consumption in Nigeria accounts for 40% of the total vegetable consumed per day [1,2]. Large scale pepper production in Nigeria is mostly done in the northern part of the country under irrigation system during the dry season (September-March). Pepper varieties (Capsicum annuum abbreviatum and grossum) are economically important spices and cultivated mainly to improve the palatability and the visual appearance of diets. The most popular are Capsicum annuum, the large-fruited sweet pepper (grossum), the medium corrugated fruited hot pepper (abbreviatum) and the small fruited pepper. In these three groups, variation exists in fruit shape, size, maturity period and quantity of yield [3]. Pepper grown in Nigeria is in high demand because of its pungency and good flavor. It can be readily dried, ground and packaged for export. Traditional drying methods (sun drying) are known to be associated with microbial proliferation and quality deterioration [4]. The process is usually lengthy taking up to 4-10 days to reduce the moisture content to 9.9% [5]. Since pepper is susceptible to fungal proliferation, this process creates favorable conditions for mycotoxins contaminations. In order to prevent fungal proliferation and improve the quality different drying methods have been employed. Pepper fruits can be considered carotenogenic fruits due to the richness of the carotenoid profile within them, which contains over 20 different carotenoids. [6,7]. Carotenoids control pod colour and the major red colour in pepper comes from the capsanthin and capsorubin, which are unique to pepper while the yellow-orange colour is from β-carotene and violaxanthin. Capsanthin in ripe pepper fruits, contributes up to 60% of the total carotenoids. Capsanthin and capsorubin increases proportionally with advanced stages of ripeness, with capsanthin being the more stable of the two. The colour of pepper is due to the presence of red-pigmented carotenoids. However, when pepper is dried and grinded into powder or oleoresin extracted, the carotenoids auto-oxidize, due to the effects of heat, light and oxygen. This leads to a more orange and less intense red coloration that devalues the product. This study therefore investigated the impact of drying on the carotenoid, surface and extractable colours of the species Capsicum annuum grown in selected locations of Oyo state in Nigeria.
2. Materials and Methods
2.1. Materials
- Organically grown matured pods of C. annuum var abbreviatum and C. annuum var. grossum were harvested from farm locations in Ogbomoso, Ibadan and Eruwa in Oyo state in Southwestern Nigeria. Five kilograms of each variety from the locations were mixed and stored at 4°C for two days. Ten kilograms from each variety were subjected to sun and cab- net drying. All reagents and solvents used were of analyticalgrade.
2.2. Preparation of Samples
- Fresh peppers namely C. annuum var abbreviatum (AA) and C. annuum var. grossum (AG) were washed and destalked. The pod was blanched using hot water at 90°C for 3 min (Anoraga et al., 2018) cooled in cold water and drained on a perforated tray before drying.
2.3. Sun Drying
- Sun drying (SD) of fresh pepper samples was carried out using the method of Maurya et al. [8]. Pepper samples blanched at 90°C for 3 min, spread in a single layer and exposed to sunlight varying between 33.6 to 37.5°C and an average humidity varying from 45% to 68% until constant weights was obtained. Sun dried samples of C. annuum var abbreviatum (SAA) and C. annuum var. grossum (SAG) cooled to room temperature and packaged in airtight bags.
2.4. Cabinet Drying
- Cabinet drying of fresh pepper samples was obtained following the method of Maurya et al. [8]. The blanched pepper was placed on perforated trays with an area of ≈ 0.2 m2 and dried at 60°C for 24 h using a cabinet drier (Serwell Instruments Inc., Bangalore, India; 55 L volume). Cabinet dried (CD) C. annuum var abbreviatum (CAA) and C. annuum var. grossum (CAG) were cooled to room temperature and packaged in airtight bags.
2.5. Moisture Content (Mc) And Water Activity (Aw)
- Moisture content (Mc) of fresh and dried pepper samples were determined using AOAC [9] method. The water activity (aw) of the samples were measured at 25°C with a water activity meter (Aqua Lab Series 3TE, Pullman, WA, USA).
2.6. Determination of Carotenoid Profile
- Fresh and dried pepper samples were homogenized, weighed and subjected to carotenoid extraction as described by Mınguez-Mosquera and Hornero-Mendez [10]. After saponification, the organic layer of the extract was vacuum-dried. HPLC quantification of carotenoid was carried out with Rayleigh-LC100 system equipped with Surveyor Auto Sampler Plus, Surveyor LC Plus quaternary pump and a surveyor photodiode array (PDA) detector. The chromatographic conditions were: Betasil C18 column (particle size 3 μm, and dimension 250mm × 4.6×0.5), 25°C column temperature, 9.24 mg/mL sample quantity, 460 nm UV detection wavelength. The mobile phase was a mixture of acetonitrile and water (75:25 v/v) and a flow rate of 1.5 mL/min. A 20 μL aliquot was used for each HPLC injection. Capsanthin, capsorubin and violaxanthin were determined by comparison with external standards from Sigma-Aldrich (St Louis, Missouri, USA). Carotenoid identification was based on retention time measured under identical HPLC conditions, while quantitative determination in the pepper samples was achieved using the peak areas.
2.7. Extractable Colour Determination
- Extractable colour of fresh and dried pepper samples were determined according to American Spice Trade Association ASTA, [11] method with some modification as follow. Sample (5 g) was weighed into about 100 mL acetone in 250 mL screw-cap jar kept in the dark for 16 h at ambient temperature. The jar was axially shaken at 140 rpm under ambient condition. Then the extract was diluted 1/5 with acetone. The absorbance of the diluted extract was measured against acetone at 460 nm by spectrophotometer (Shimadzu UV160 U). The extractable color of the samples was expressed in ASTA units (Eq.1).
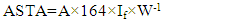 | (1) |
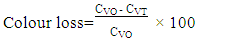 | (2) |
2.8. Colour Measurement
- Colour measurements of fresh and dried pepper samples was determined using a Hunter-Lab Colorimeter (Miniscan XE Plus 4500. The instrument (45° /0° geometry, D 65 optical sensor and 10° observer) was calibrated with black and white reference tiles through the tri-stimulus values X, Y, Z, taking as standard values those of the white background (X = 79.01; Y = 83.96; Z = 86.76) tile. Glass cell containing the samples was placed above a light source and colour values namely L, a, and b were recorded in triplicate and average values determined. The total colour change (ΔE) (Eq 3), chroma (Eq. 4), hue angle (Eq. 5) and browning index (Eq. 6) were calculated from the Hunter scale and used to describe the colour change during drying:
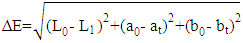 | (3) |
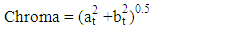 | (4) |
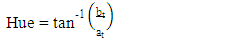 | (5) |
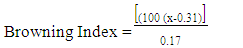 | (6) |
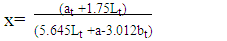 | (7) |
2.9. Statistical Analysis
- Data analyses was done using statistical package for social sciences software (SPSS, IBM, version 26). Triplicate data were analyzed using one-way analysis of variance (ANOVA) and differences within the means determined by Duncan multiple comparison test at a significance level of p<0.05.
3. Results and Discussions
3.1. Moisture Content (Mc) and Water Activity (aw)
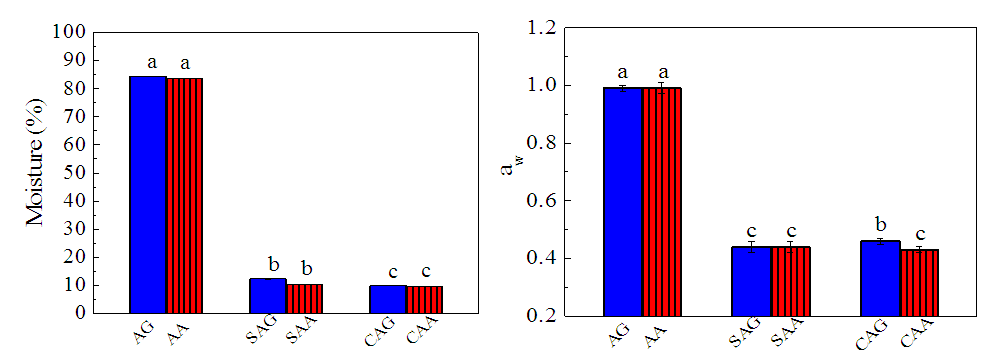
- The drying methods affected the moisture (Figures 1a) and water activity (Figures 1b) of the C. annuum var abbreviatum and C. annuum var. grossum. Cabinet drying was better in terms of moisture removal than the sun drying method as the dried (CAA, CAG) had lower moisture content and water activity compared to the sun dried (SAA, SAG) samples. Similar results were recorded by Vega-Galvez et al. [12] with dehydrated hot pepper stored under various conditions. Colour change in red pepper powder depends on temperature and aw, as these value increases so does the color increasingly fades to become brown and tarnish black. This may be attributed to the degradation of carotenoid pigments and development of browning compounds [13]. Water activity of 0.4 is required in most fruits and vegetables to minimize microbial growth, chemical and enzymatic reactions in order to get a good storage stability [14]. The water activity of all the dried pepper samples (CAA, CAG, SAA, SAG) which varied between 0.42 and 0.45 were in the range that will not encourage deteriorative changes that could affect the pepper quality if properly stored.
3.2. Effect of Drying Methods on Carotenoids Content
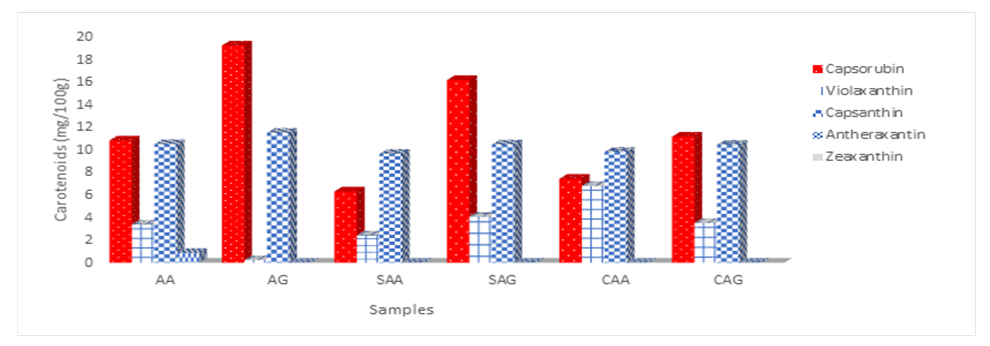
- Capsanthin and capsorubin (11.48 mg/100 g and 19.19 mg/100 g) were the predominant carotenoid in the fresh (AA, AG) pepper samples with C. annuum var. grossum having 9% and 44% higher values compared to the C. annuum var. abbreviatum as shown in Figure 2. Capsorubin, violaxanthin and capsanthin values significantly differs (p<0.05) in sundried samples with 6.30, 2.41 and 11.48 mg/100g in SAA while 16.15, 4.07 and 10.45 mg/100g was obtained in SAG. Antheraxanthin and zeaxanthin was not detected in the sundried samples. Capsorubin loss was 42.9 and 15.8% in SAA and SAG while capsanthin loss was 10.4 and 8.9% respectively. Cabinet dried samples also differs significantly (p <0.05) with CAA having 7.42, 6.78 and 9.79 mg/100g capsorubin, violaxanthin and capsanthin, while 11.14, 3.51 and 10.42 mg/100g was recorded in CAG. Capsorubin losses due to cabinet drying was 29.1 and 41.9% in CAA and CAG while capsanthin loss was 6.6 and 0.57%. The carotenoid values recorded in this work is within the range reported by Pinar et al. [15], Topuz & Ozdemir, [16] and Mohd-Hassan et al. [17]. Capsicum annuum var grossum exhibited better colour retention with the drying methods than C. annuum var abbreviatum. Antheraxanthin and zeaxanthin was not detected in the cabinet dried samples. There was relatively higher loss of capsorubin than capsanthin with cabinet drying compared to sun drying. The lower drying temperature and longer drying time during sun drying prolonged the duration for degradation and oxidation of carotenoids. According to Mohd-Hassan et al. [17] the concentration of capsanthin during open-sun drying decreased significantly which may be associated with duration of exposure, temperature and sunlight. The fading colour, which appears upon prolonged exposure to temperature and light, was due to isomerization reactions shifting from trans to cis configurations [18]. Figueroa-Garcia et al. [19] reported a reduction in capsorubin and capsanthin in Capsicum chinenses species irrespective of varietal differences across the samples. The temperature and time required in both drying processes, depends on the variety, environmental conditions, and stage of maturity of the pepper samples. In some varieties, shorter drying time at high temperature are favorable, while others present greater losses. Report by Yang et al [20] indicated that the activated at around 60°C causing further degradation [21]. According to Topuz et al. [22] violaxanthin, zeaxanthin, capsolutein, β-cryptoxanthin and β-carotene are mainly carotenoids that provide the yellow colour in pepper, the high degree of unsaturation of these compounds makes them susceptible to oxidation and degradation during thermal drying, thus resulting in the loss of colour and subsequently a reduction in their values with cabinet drying. Nevertheless, the decomposition of carotenoids occurs easily through oxidation, isomerization, and fragmentation of their molecules, particularly when exposed to improper processing and storage conditions [6]. Therefore, controlling the degradation of this pigment could improve dried pepper market value. The surface colour appearances of red chilies are related to carotenoid contents, particularly capsanthin compound, which are the main red pigment [23].
3.3. Capsanthin to Capsorubin (Caps: Caps) Ratio in Fresh and Dried Pepper Samples
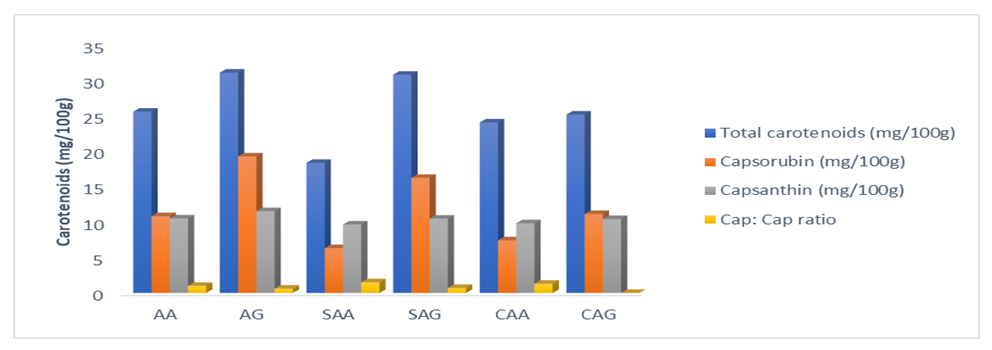
- Capsanthin and capsorubin are major carotenoids peculiar to capsicum species. The total carotenoid in fresh (AA, AG) pepper samples were 25.52 and 30.90 mg/100g.The caps: caps ratio was higher (0.97) in C. annuum var. abbreviatum than C annuum var. grossum (0.67). This implies the difference in concentration of capsanthin and capsorubin is smaller compared to the difference in C. annuum var. grossum. The capsanthin to capsorubin (caps: caps) ratio was affected by the drying methods as shown in Figure 3. A progressive increase was recorded with drying methods as the ratio in the dried samples significantly differs (p < 0.05) with 1.52 and 0.65 in SAA and SAG and 1.33 and 0.92 in CAA and CAG. The C. annuum var. grossum still had a lower ratio compared to the C. annuum var. abbreviatum, this result indicated a higher disparity in the capsanthin and capsorubin concentration in the C. annuum var abbreviatum. Though C. annuum var. grossum had higher concentration of the carotenoids, but with a lower disparity in their value hence the smaller ratio. Capsanthin contributes 30–70% of carotenoids change of red pigments content was dependent on both the drying time and temperature. Furthermore, the lipoxygenase, which was an aerobic catalyst of oxidation reactions, was in most of the varieties and cultivars, the distribution is however affected by agronomic practices [24,25]. Carotenoid esters, particularly monoesters in pepper (C. frutescens) and diesters in paprika (C. annuum) samples, showed higher stability than the non-esterified pigments [26]. However, the susceptibility of the mono- and diesters belonging to the capsanthin, zeaxanthin, capsorubin and β-cryptoxanthin esters showed comparable processing stability because higher stability of the esterified pigments seemed to be related to their more lipophilic nature and hence, to their better integration into membrane structures, which might protect the carotenoids from thermal degradation. The relatively higher capsanthin concentration in dried SAG and CAG may be associated with the esterified nature of the capsanthin in the species. The structural diversity of carotenoids also produces variable rate of oxidation, resulting in non-uniform degradation depending on their functional groups. Susceptibility of the esterified carotenoids was found to differ, with capsanthin and β-cryptoxanthin retaining higher initial carotenoid. As reported in a previous study carotenoids esterified with lauric, myristic and palmitic acids exhibited a lower susceptibility to degradation than their non-esterified forms because esterified pigments with their content of saturated fatty acids are more stable, being less prone to photo- and thermo-oxidative degradation [24]. Available literature on processing and storage stability of carotenoid esters are scarce and sometimes contradictory. The results of this study confirmed a lower susceptibility of capsanthin to thermal degradation during drying.
3.4. Effect of Drying on Extractable Colour
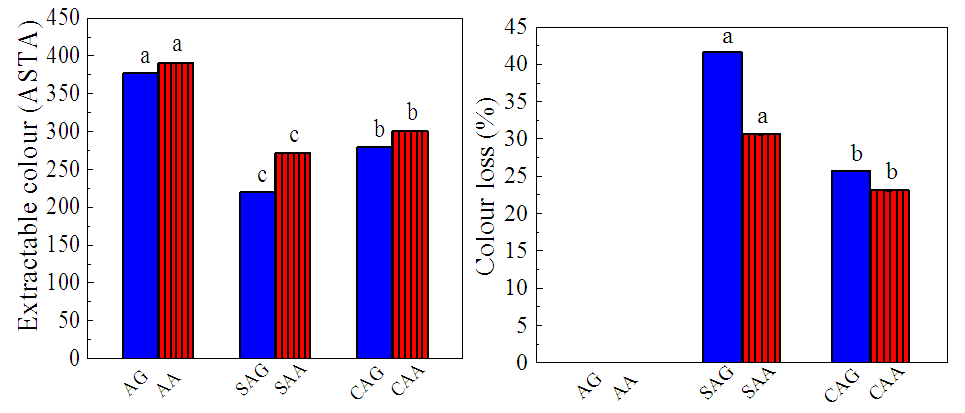
- This study showed that the ASTA colour values significantly (p<0.05) changed by the different drying methods as shown in Figure 4a. Extractable colour is generally expressed as ASTA colour value which is a quality parameter representing the total pigment concentration. Extractable colour value for the fresh pepper (AA, AG) samples were 377.2 and 390.8 while the values for the sundried (SAA, SAG) were 220.2 and 271.3, respectively indicating a lower extractable colour values compared to the fresh samples. The values 280.2 and 300.6 were recorded for cabinet dried (CAA, CAG) samples. Factors such as cultivar, temperature, methods of drying, oxygen/air in the atmosphere, and moisture content during storage affect the retention of colour [27,28]. In this study there were significant differences in the extractable colours with methods of drying as the sundried (SAA, SAG) had 41.6 and 33.3% loss compared to the cabinet dried (CAA, CAG) with 25.7 and 23.1% respectively indicating a better colour retention when compared to the sundried samples (Figure 4b). Generally, the higher the ASTA colour the more intense and acceptable is the pepper. Capsicum with 200 ASTA colour units and above would give a brighter red colour to a finished product than an equivalent amount with 100 ASTA colour units [29].
3.5. Effect of Drying on the Chromatic Coordinates of Pepper
3.5.1. Colour Redness, Yellowness and Lightness
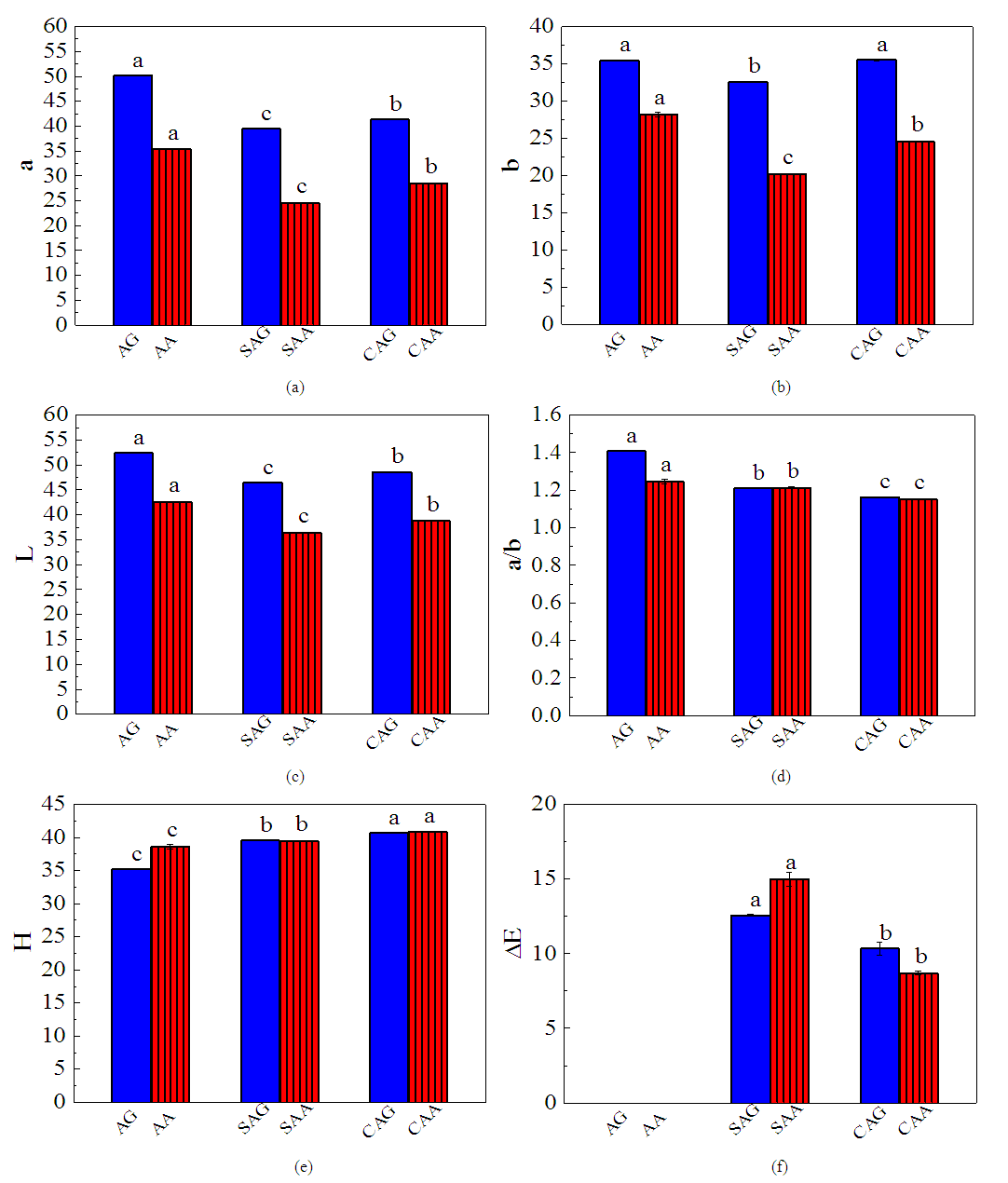
- Redness (a*), yellowness (b*), and lightness (L*) were affected by the drying methods as shown in Figures 5a to 5c. After sun drying, the redness in the fresh (AA, AG) samples decreased by 32% and 21% while yellowness (b*) decreased by and 8%. The C. annuum var grossum exhibited significantly (p< 0.05) higher colour retention irrespective of the drying methods as opposed to the C. annuum var abbreviatum. This may be due formation of brown pigments as a result of the drying temperature [30]. The lightness (L*) also decreased with sun and cabinet drying methods with C. annuum var grossum having higher value (Figure 5c). Since L* is a measure of the colour in the light-dark axis, value indicated that the samples were turning darker. It has been observed that the variation in the brightness of heat-treated samples can be taken as an indication of browning [31]. This was also reported by Swain et al. [32] which was possibly due degradation of thermolabile pigments resulting in formation of dark compounds that reduced luminosity. Discolouration of samples during drying by any technique may be related to pigment destruction, ascorbic acid browning, and non-enzymatic Maillard browning.
3.5.2. The Effect of Drying on the Redness to Yellowness (a/b) Ratio
- The redness to yellowness (a/b) values for the fresh (AA, AG) pepper samples were significantly different with the C. annuum var. grossum showing a higher value compared to the C. annuum var. abbreviatum (Figure 5d). The drying methods also resulted in significant reduction with the sun dried samples (SAA, SAG) recording higher losses compared to the cabinet (CAA, CAG) samples which may be associated with heat-induced degradation of color pigments.
3.5.3. Hue Angle
- The hue angle (H) of pepper increased by the drying methods (Figure 5e). After sun drying, H values in fresh (AA, AG) samples increased by 2% and 12% while after cabinet drying H values increased by 6% and 15%. Hue angle has been extensively used to characterize and evaluate colour parameters in food products (green vegetables, fruits, and meats). The increasing trend in hue angle in this study may be attributed to greater degradation of red and generation of yellow colour (zeaxanthin) as a result of drying leading to orange colour products. This trend is in agreement with the values obtained for kiwi fruit [33] and okra [34] using microwave drying method.
3.5.4. Total Colour Difference (ΔE)
- Total colour difference (ΔE) represents the overall surface colour change as it is a function of the chromatic coordinates. The ΔE values of cabinet dried was significantly lower than the sun-dried red pepper (Figure 5f) this implied a better colour retention because of the lower values compared to the sun dried. Degree of colour difference between two samples due to total colour change can be interpreted in the following way: ΔE in the range of 0–0.5 indicates an imperceptible difference in colour, 0.5–1.5 a slight difference, 1.5–3.0 a just noticeable difference, 3.0–6.0 a marked difference, 6.0–12.0 an extremely marked difference, and above 12.0 a colour of a different shade [7].
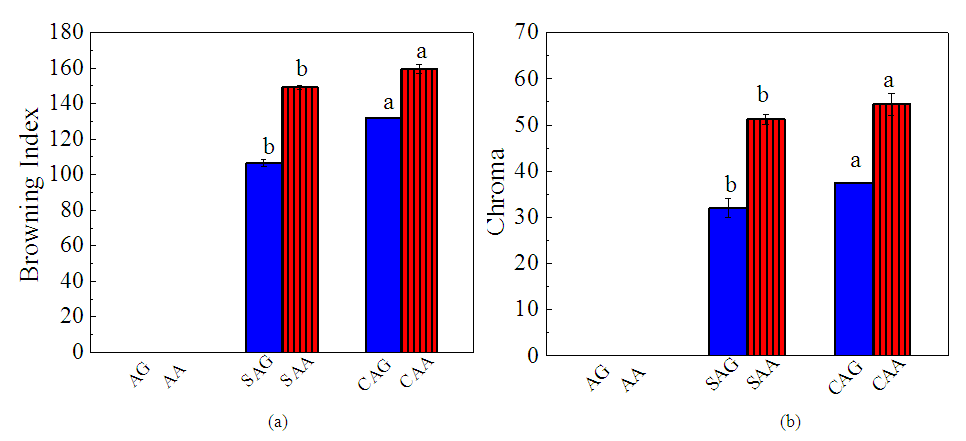
3.5.5. Browning Index (BI) Values of Dried Pepper Sample
- Values of BI, as shown in Figure 6a increased with cabinet drying in both samples with CAG having the highest BI value. Lower value of BI in sundried pepper samples indicated little changes associated with enzymatic browning consequent of the relatively lower drying temperature. Some authors reported that brown pigment in dried red peppers was due to their high levels of reducing sugars and amino acids [35,36] Browning index signifies the purity of brown colour and an important parameter in processes where enzymatic and non-enzymatic browning takes place [37]. These reactions occur when polyphenol-oxidase (PPO) and/or peroxidase (POD) stimulates oxidation reaction of phenolic compounds (colourless) to quinones that have auburn colour [25]. There were reports that thermal processing does not completely inactivate the enzyme activities, except those with a temperature over 80°C [38]. Consequently, during the sun and cabinet drying carried out on the pepper samples the enzymes are still active and may initiate the colour change especially in the cabinet drying method under high humidity, which impact the colour of the pepper product. The values and justification from this work contrast Kumar et al. [39] who reported that the short drying time of Refractance Window Drying (RWD) method suppressed the browning reaction and the longer duration of 4–5 days under direct sunlight.
3.5.6. Chroma Value
- The chroma (C) value of pepper decreased during drying process (Figure 6b). However, C values of SAG and CAG were 38% and 31% higher than those of SAA and CAA. Chroma indicates the degree of saturation which is proportional to the strength of the colour. The higher values in C. annuum var. grossum indicated a greater colour saturation. This implies that the chroma values of the grossum variety remained more stable with sun and cabinet drying methods. These values and trend are comparable with the work of Getahun et al. [40] in drying characteristics and quality attributes of chili pepper at different maturity stages there was a change in total colour difference due to drying. The surface colour in CAA and CAG were closer to samples AA and AG, while sun drying method significantly altered the surface colour of the final products, indicating that cabinet drying may be considered a better drying alternative for dehydration of red pepper due to better colour retention. Prolonged drying time due to sun drying led to higher ΔE values. This result is consistent with a previous findings that temperature and duration of drying were important factors for colour degradation during drying [25]. Furthermore, browning reaction and carotenoid changes were considered as the main causes of the surface colour changes, since the trends of browning index (BI) and extractable colour were consistent with the ΔE value changes [20,30]. Part of the reason for this may be that some other components apart from browning substances and carotenoids, such as vitamins, carbohydrates and amino acids in the material affect the final product colour [25].
4. Conclusions
- Pepper cultivar, drying duration and methods significantly affected carotenoids and colour characteristic of C. annuum. The cabinet drying method was better in terms of moisture removal hence the cabinet dried samples had lower moisture content and water activity compared to the sun dried. Capsorubin losses was however higher than capsanthin loss due to cabinet drying in both varieties. The sundried pepper indicated higher extractable colour loss than the cabinet dried pepper samples. The C. annuum var grossum exhibited significantly higher colour retention capacity irrespective of the drying methods compared to the C. annuum var abbreviatum. Values of BI increased with cabinet drying in both samples with the cabinet dried C. annuum var grossum having the highest chroma values. To maintain good colour quality, high carotenoid retention and low browning, C. annuum var grossum should be considered at water activity not higher than 0.5 and cabinet drying was observed to be most efficient in minimizing colour loss.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML