-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2023; 13(1): 1-8
doi:10.5923/j.food.20231301.01
Received: Jun. 17, 2023; Accepted: Sep. 15, 2023; Published: Sep. 22, 2023

Impact of an Aerobic Training Program Alone or Combined with Nutritional Education on Pro/Inflammatory Cytokine Levels in Obese Adolescents
Simplice Innocent Moussouami1, 2, Agbodjogbe Kpèdétin Wilfrid Dieu Donné3, Eddie Janvier Bouhika2, Yvon Rock Ghislain Alongo2, Misère Emmanuel Rodrigue Loubota2, Bio Nigan Issiako1, François Mbemba2
1Sport, Health and Evaluation Research Unit (UR/SSE), National Institute for Youth, Physical Education and Sport (INJEPS), University of Abomey-Calavi (UAC), Porto-Novo, Benin
2Laboratory of Physiology of Effort and Biomechanics (LPB), Higher Institute of Physical Education and Sport (ISEPS), University Marien Ngouabi, Congo
3Laboratory of Physiology of Effort, National Institute for Youth, Physical Education and Sport (INJEPS), University of Abomey-Calavi (UAC), Porto-Novo, Benin
Correspondence to: Simplice Innocent Moussouami, Sport, Health and Evaluation Research Unit (UR/SSE), National Institute for Youth, Physical Education and Sport (INJEPS), University of Abomey-Calavi (UAC), Porto-Novo, Benin.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Obesity is associated with low-grade inflammation. Several training modalities are proposed to combat obesity but also to improve the inflammatory profile. The aim of this study was to evaluate the effects of physical activity combined with nutritional education on pro/inflammatory cytokines in obese adolescents. Methods: In this intervention study, 44 adolescents aged 15 ± 1.38 were selected. Participants were randomly separated into two groups: group 1 (n = 22) who completed the training program and nutrition education; group 2 (n = 22), the training program alone. Anthropometric and physiological measurements were performed. All subjects underwent assessment of inflammatory markers before and after the 12-week training session with measurements of (C-reactive protein) CRP interleukin-6 (IL-6) and tumor necrosis factor-α, (TNF-α). Results: The results indicated a significant decrease in body mass (0.78%) waist circumference (4.33%) and fat percentage (4.82%) in group 1 subjects. Those in group 2 also showed a significant difference in waist circumference (1.91%) and fat percentage (0.61%). Significant decreases in serum levels of TNF-α (p < 0.05), IL-6 (p < 0.05) in both groups were observed after the intervention. However, only group 1 showed a decrease in CRP (p = 0.04). Conclusion: This study showed that serum levels of TNF-α and IL-6 and CRP can be reduced in obese adolescents after 12 weeks of exercise training combined with diet modification. Exercise combined with dietary education is an important therapeutic aspect to increase success in combating the harms of obesity.
Keywords: Aerobic exercise, Nutrition education, Obesity, Inflammatory markers
Cite this paper: Simplice Innocent Moussouami, Agbodjogbe Kpèdétin Wilfrid Dieu Donné, Eddie Janvier Bouhika, Yvon Rock Ghislain Alongo, Misère Emmanuel Rodrigue Loubota, Bio Nigan Issiako, François Mbemba, Impact of an Aerobic Training Program Alone or Combined with Nutritional Education on Pro/Inflammatory Cytokine Levels in Obese Adolescents, International Journal of Food Science and Nutrition Engineering, Vol. 13 No. 1, 2023, pp. 1-8. doi: 10.5923/j.food.20231301.01.
Article Outline
1. Introduction
- Obesity is an important multifactorial condition associated with risk of many chronic diseases and high mortality rates [1]. It is characterized by abnormal or excessive accumulation of adipose tissue that can have adverse health consequences for individuals of all ages. The global obesity phenomenon has worsened in the last decades [2] and affects both adults and adolescents. The increase in the prevalence of adolescent obesity has been attributed to an imbalance between energy intake and expenditure due to an increasingly sedentary lifestyle, and a nutritional shift towards processed foods and high-calorie diets over the past 30 years [4].Adolescence is a key period in the development of obesity observed in adulthood. Indeed, at this period, regardless of gender, hormonal impregnation induces an increase in body fat, increased by the decrease in physical activity often observed in adolescents. Fat mass gain favors the development of insulin resistance (IR) and chronic inflammation, well known factors of oxidative stress (OS) at rest [3]. In addition to being a risk factor for cardiovascular disease, obesity is associated with a large number of abnormalities such as hypertension, metabolic syndrome, certain cancers, sleep apnea syndrome and type 2 diabetes [5,6]. It is also associated with low-grade inflammation independent of body mass index. Indeed, excess fat cells in obese subjects release more inflammatory cytokines such as C-reactive protein (CRP), tumor necrosis factor alpha (TNF-) and interleukin 6 (IL-6) and raise the level of oxidative stress. This leads to insulin resistance in skeletal muscle that may result in diabetes [7]. Circulating levels of these inflammatory markers (CRP, TNF-α and IL-6) are then elevated with obesity [8].Due to the complications of obesity, the prescription of physical activity in obese adolescents is no longer a rarity and is therefore recognized as a necessity. It is considered to be an effective therapeutic approach in the fight against chronic diseases. Previous studies have indicated that regular physical training such as aerobic or resistance training reduces chronic inflammation, particularly in obese subjects with elevated levels of inflammatory markers undergoing longer- term intervention [9]. Kim et al [10] concluded that circuit training can be considered an effective mode to help decrease blood inflammatory factors in the obese person.Another aspect that could accelerate the effect of exercise on weight loss and consequently the improvement of serum levels of inflammatory markers in the obese adolescent is the adoption of healthy eating habits. Indeed, nutrition education would be an optimal tool to improve dietary habits, which could have implications on inflammatory parameters in obeses adolescents.Some studies on the effects of high-intensity interval training ("HIIT") and nutritional counseling on cardiometabolic biomarkers, hormonal parameters, and cardiorespiratory fitness in adolescent girls with obesity have shown that after three months of intervention, the diet+HIIT group showed a decrease in the area under the curve of glucose, insulin, and CRP compared to the diet group [3].However, other recent studies have not confirmed the effects of exercise on inflammatory cytokines [12] and probably due to the non-isolation of the effects of exercise-induced weight loss. Furthermore, the mechanism linking exercise to these inflammatory biomarkers is not clearly defined and the effects of regular exercise on serum levels of pro/inflammatory cytokines are not conclusive. The nature of the intervention may be important in inducing favorable changes in body composition and inflammatory markers in the obese individual. Therefore, more studies are needed to show the importance of a type of intervention on health markers in this population. Therefore, the purpose of the present study was to compare the effects of 12 weeks of an aerobic training program combined with nutrition education to an aerobic training program alone on pro/inflammatory cytokines.
2. Materials and Methods
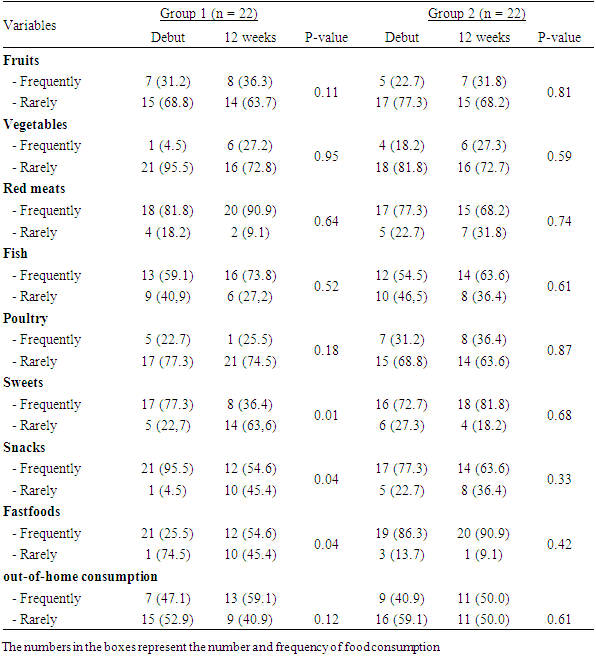
- ParticipantsThe purpose of this quasi-experimental research study was to evaluate the effectiveness of two types of training programs on pro/inflammatory cytokines. A total of 44 obese adolescents aged 14 to 18 years were recruited for this intervention study. Parents of all subjects were informed of the experimental procedures and provided written informed and signed consent prior to any data collection. Eligible study subjects were randomized into two groups: group 1 (n = 22) who completed the training program and nutrition education; group 2 (n = 22), the training program only. Obesity was defined with a body mass index (BMI) ≥ 95th percentile. Any subject with no history and type of medical condition that precluded physical activity was included in the study.The study was approved by the scientific committee of the Higher Institute of Physical and Sports Education. Parents of children younger than 18 years of age signed an informed consent before participating in the training session.ProceduresAll subjects were familiar with all procedures. Assessment of anthropometric and body composition parameters, measurement of serum inflammatory markers (TNF- α, IL-6, and CRP), and dietary habits were performed before and after the 12-week training session.Anthropometric and body composition parametersBody mass (kg) was measured to the nearest 0.1 (kg) with the participant in light clothing and without shoes using a digital scale (SECA707, Hamburg, Germany). Body height (m) was determined to the nearest 0.5 cm using a stadiometer (Takei, Tokyo, Japan). Body mass index (BMI) was then calculated (kg/m2). Waist circumference was measured twice using a flexible anthropometric tape placed on the upper edge of the iliac crest and recorded to the nearest centimeter. Fat percentage was measured using a BC535 multifunction impedance meter (Tanita, Japan).Biochemical analysisBlood samples from all subjects were taken one day before and two days after their last exercise session. This blood was allowed to clot for 60 minutes, centrifuged at 1000 rpm, (4°C), and the serum was collected and stored at -20°C until inflammatory biomarker analysis. Blood concentrations of IL-6, TNF- and CRP were measured in double assay by enzyme-linked immunosorbent assay according to the manufacturer's specifications.Dietary habitsThe dietary habits of the children were collected by a food frequency questionnaire (FFQ). The food frequency questionnaire included the following food products consumed most often in Congo: soft drinks, fruits, vegetables, red meat, fish, sweets. It was divided into frequency per week (rarely, frequently). In addition, 2 other questions were added separately to the FFQ regarding the frequency of consumption of fast-food type meals.Intervention ProgramThe training program was structured in two microcycles of 18 sessions each. The duration of each microcycle was six weeks. Three weekly sessions were performed during this program mainly on Monday, Wednesday and Friday. Each session was 1 h 30 min in length. The sessions consisted of six different parts, namely: 1) 5 min of grip, 2) 15 min of warm-up, 3) 30 min of muscle strengthening, 4) 25 min of aerobics, 5) 10 min of relaxation, and 6) 5 min of cool-down and recovery.During microcycle 1, subjects worked at 65% of HRmax from week 1 to week 4, then from week 5 to week 6 the intensity was slightly increased to 70% of HRmax. In microcycle 2, the intensity in weeks 7 and 8 was also 70% of HRmax. This intensity was increased to 80% of HRmax from weeks 9 to 12.During the sessions, the warm-up part aimed at cardiovascular activation, joint mobilization and muscle warm-up. It was carried out using fast walking, slow running and jumping exercises followed by stretching.The muscle strengthening sequence was organized as a circuit. This circuit included 12 workshops that can be grouped mainly into squats, push-ups, sit-ups, crunches and jumps. The squats, push-ups and sit-ups were performed in 2 sets with 30 seconds of recovery. Each set consisted of 10 repetitions for the first 4 weeks, 15 repetitions for weeks 5-8, and 20 repetitions for the last 4 weeks. Cladding were also performed in two sets separated by 30 seconds of recovery. The set includes 20 seconds of effort for the first 4 seconds and 30 seconds for the last 8 weeks. During the cycle, improvements in aerobic capacity and endurance strength were targeted at the energy and muscle levels, respectively.The nutrition education component of the weight management program was based on a non- dieting approach, which aimed to bring about changes in participants' eating habits. A total of 24 sessions made up this program with two weekly sessions of one hour each. Themes covered in the first 6 weeks included "drinking healthy beverages (avoiding unhealthy soft/sweet drinks)"; "increasing consumption of vegetables and legumes"; "reducing consumption of sweets and pastries while increasing fresh fruits and nuts"; and "increasing consumption of fish". From week 7, the following topics were covered: consequences of poor food choices; food rhythm; food pyramid. The registered dietitians used the Smart Moves manual, which provided a consistent structure for all session topics.The objectives and content of the nutrition education training sessions are summarized in Table 1.
|
3. Results
- The anthropometric characteristics, body composition and serum levels of inflammatory parameters before the intervention of the two groups were compared in table 2. The two groups were identical with respect to the age of the participants (16.00 ± 1.41 vs. 15.54 ± 1.37 years), body mass (75.14 ± 6.63 vs 73.70 ± 5.25 kg), body mass index (31.72 ± 1.61 vs 31.57 ± 1.21 kg/m2). The post intervention blood concentrations of TNF-α (3.05 ± 0.05 vs 3.09 ± 0.09 pg/ml), and CRP (2.36 ± 0.06 vs 2.38 ± 0.02 mg.L- 1) between group 1 (exercise program combined with nutrition education) and group 2 (exercise program alone) showed no significant difference apart from IL-6 level which showed a significant difference (P = 0.018).
|
|
4. Discussion
- The present study compared the effects of two types of intervention on pro/inflammatory cytokine levels in obese adolescents. The first is based on an aerobic training program combined with nutritional education and the second on an aerobic training program only. In group 1, the 12-week program resulted in a significant decrease in body mass and fat percentage compared to baseline. But there was also a decrease in serum levels of pro-inflammatory cytokines such as IL-6 and TN-α in both groups at the end of the programs. This study also showed that in adolescents who followed a combined program, the intervention had positive effects on the level of inflammatory cytokine (CRP).Overall, the data from this study showed a significant decrease in body composition parameters after the combined intervention. The magnitude of this decrease was at least 4%. In this context, Luo et al [13] observed a reduction in body mass fat percentage of 3.7% after an intervention period based on a yoga program combined with a 12-week aerobic training program. Similarly, some authors have shown that regular exercise could contribute to the improvement of certain parameters of body composition by decreasing, for example, the amount of fat that is considered an important source of inflammation [14]. If no change in body composition was observed in obese people who followed the aerobic program alone, then it can be hypothesized that the loss of body mass and percentage of fat observed following the aerobic program combined with nutrition education is due to dietary restriction after a change in eating behavior and significant water loss caused by the intensity and duration of the exercise. Another potential reason for this improvement could probably be related to the result of upregulation of bioenergetic oxidation and thus an increase in lipid oxidation.It has also been shown that weight loss can lead to an improvement in the pro/inflammatory profile, as was the case in this study. Indeed, the beneficial effects of exercise on pro- inflammatory cytokine levels observed after the two types of intervention proposed in this study are not surprising since the majority of results from the literature demonstrate a reduction in plasma concentrations of several cytokines in response to high-intensity programs [15,16].The results of many studies are consistent with those obtained in the present study. Indeed, the decreases in cytokines observed in these studies would certainly be due to the loss of weight and body fat following 12 weeks of the proposed intervention programs. In this regard, Beavers et al [9] reported that reductions in inflammatory cytokines are not achieved by exercise, but are directly associated with fat loss. This suggests that significant reductions in inflammatory cytokines can be achieved with a fat mass loss greater than 5%.The decrease in IL-6 and TN-α cytokines observed in this study is similar to the study by some authors. Steckling et al [16] observed a significant decrease in plasma concentrations of resisting, leptin, IL-6 and TNF-α after an intervention based on a high intensity interval exercise program. Several authors have reached the same results [17,18]. These results from previous and current studies support why obese adolescents should exercise regularly and adopt healthy eating habits to alter body composition. Indeed, it has been reported that IL-6 levels rise in the obese and are positively associated with a waist circumference ratio and BMI, and decrease with weight loss [18].Surprisingly, although a decrease in the levels of the proinflammatory cytokines IL-6 and TNF- α was observed in both groups, this finding was not the same for the exercise-only group where CRP levels did not significantly change. Consistent with the literature, the study by Koh and Park [19] observed a significant decrease in plasma concentrations of some markers, independent of a change in body composition. In addition, Arsenault et al obtained no change in the inflammatory profile with training at 50% of VO2 max for different training durations in one of the overweight or obese subjects [20]. The present results are also contradictory to those of the study by Nunes et al [21] who reported an increase in plasma IL-6 levels after 12 weeks of high intensity interval training.These trends can certainly be explained by the fact that in these studies, the dietary behavior of the subjects was not strictly followed, and they were not put on a diet that would allow them to lose weight. Another plausible explanation for these discrepancies is that the duration, intensity and type of exercise offered in these studies were different from one study to another. Several mechanisms could account for the decrease in CRP levels following the aerobic program combined with nutrition education. The primary mechanism by which physical activity may modulate inflammation levels is via muscle-derived cytokines (myokines) released from concentrated skeletal muscle. It is therefore highly likely that the benefits of this training are due to the use of significantly elevated muscle mass during exercise [22], which increases myokine production. The observation that serum CRP levels did not decrease in the aerobic training group alone is consistent with other studies showing that concomitant weight loss is required before a decrease in CRP levels becomes apparent [23].There were several limitations to the present study: Firstly, the sample size was limited because the study was conducted in a single locality and in a few local schools. Similarly, most of the adolescents did not meet the inclusion criteria when they were recruited at the start of the study. Secondly, no control group was included in the study. The two groups in the study underwent different interventions and, as a result, it is not easy to differentiate the proportion of changes in the parameters studied as a result of the programs. In fact, this third group was intended to provide a baseline comparison for the effects of the interventions. Without this group, it may be difficult to distinguish the impact of the interventions from other potential influences. However, this cannot destroy the conclusions of the study for this population. Finally, the relatively short follow-up time for adolescents means that it is not possible to accurately assess the effects of this intervention over a long period. Consequently, further studies are needed to confirm the long-term effects of this multidimensional intervention.
5. Conclusions
- The results obtained suggest that the intervention program developed in this study is practically applicable in the fight against obesity in adolescents. After the interventions, the subjects who underwent the aerobic program combined with nutritional education had lower levels of inflammatory markers as well as percentage of fat than those in the group who underwent the aerobic program alone.
ACKNOWLEDGEMENTS
- We greatly appreciate all those who were involved in the data collection, as well as the participants in the project. We also thank the bioquick laboratory team for the biochemical analysis.
State of Knowledge on the Subject
- - High intensity intermittent exercise induces positive changes in body composition and inflammatory parameters.- Physical exercise combined with dietary restriction also produces positive effects on these physical and physiological parameters.
Contributions of the Study to Knowledge
- - The study showed that physical exercise combined with nutrition education produces more effective effects than physical exercise alone.- This study also set up a program for the management of obesity. This program produces convincing effects in the improvement of health parameters in obese adolescents.
Conflict of Interest
- No potential conflicts of interest were reported by the authors.
Funding
- This research was supported by the Research Program of the Marien Ngouabi University through the Laboratory of Physiology of Effort and Biomechanics (LPB), the Higher Institute of Physical Education and Sport, which provided the funding.
References
| [1] | Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017; 376: 254–266. doi: 10.1056/NEJMra1514009. |
| [2] | Mayerhofer E, Ratzinger F, Kienreich NE, Stiel A, Witzeneder N, Schrefl E et al. Multidisciplinary Intervention in Childhood Obesity Acutely Improves Insulin Resistance and Inflammatory Markers Independent From Body Composition. Front. Pediatr. 2020; 8: 52. doi: 10.3389/fped.2020.00052. |
| [3] | NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017; 390: 2627–42. doi: 10.1016/S0140-6736(17)32129-3. |
| [4] | Stamatakis E., Chau J.Y., Pedisic Z., Bauman A., Macniven R., Coombs N., Hamer M. Are Sitting Occupations Associated with Increased All-Cause, Cancer, and Cardiovascular Disease Mortality Risk? A Pooled Analysis of Seven British Population Cohorts. PLoS ONE. 2013; 8: e73753. doi: 10.1371/journal.pone.0073753. |
| [5] | Wilson PWF, Augostino RB, Sullivan L. Overweight and obesity as determinants of cardiovascular risk. The Framingham experience. Cambre. Int Med. 2002 Mar; 162: 1867-1872. |
| [6] | Corcos T. Les complications cardiovasculaires de l’obésité. Médecine & Longévité. 2012; 4(3-4): 99-110. doi: 10.1016/j.mlong.2012.10.001. |
| [7] | Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017; 127(1): 43-54. https://doi.org/10.1172/JCI88880. |
| [8] | Karstoft, Kristian; Pedersen, Bente K. Skeletal muscle as a gene regulatory endocrine organ. Current Opinion in Clinical Nutrition and Metabolic Care. 2016; (19)4: 270-275. https://doi.org/10.1097/MCO.0000000000000283. |
| [9] | Beavers KM, Beavers DP, Newman JJ, Anderson AM, Loeser RF, Jr, Nicklas BJ, Lyles MF, Miller GD, Mihalko SL, Messier SP. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthritis Cartilage. 2015; 23: 249–256. |
| [10] | Kim KB. Effect of different training modes on interleukin-6 (IL-6) and C-reactive protein (CRP) in patients with type 2 diabetes (T2D). J Exercice Nutrition Biochem. 2014; 18(4): 371- 378. doi: 10.5717/jenb.2014.18.4.371. |
| [11] | Figueiredo L, Nunes RB, Marmett B, de Sá, LB. Arbex, AK. Anti-inflammatory effects of physical exercise on obesity. Open Journal of Endocrine and Metabolic Diseases, 2017; 7(01): 44. |
| [12] | Wedell-Neergaard AS, Lehrskov LL, Christensen RH, Legaard GE, Dorph E, Larsen MK, et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metabolism. 2019; 29(4): 844-855. e3. https://doi.org/10.1016/j.cmet.2018.12.007. |
| [13] | Luo J, Zheng B. Effect of yoga combined with an aerobic exercise intervention on morphological and blood lipid indicators in female students. J. sports Med. Phys. Fit 2019; 60(3): 442-448. doi; 10.23736/s0022-4707.19.10147-8. |
| [14] | Sabag, A, Way K, Keating S, Sultana R, O'Connor H, Baker M, et al. Exercise and ectopic fat in type 2 diabetes: A systematic review and meta-analysis. Diabetes & Metabolism. 2016; 43. doi: 10.1016/j.diabet.2016.12.006. |
| [15] | Banitalebi E, Faramarzi M, Nasiri S. High-Intensity Interval Training Versus Moderate Intensity Combined Training (Resistance and Aerobic) for Improving Insulin-Related Adipokines in Type 2 Diabetic Women, Zahedan J Res Med Sci. 2018; 20(10): e68793. doi: 10.5812/zjrms.68793. |
| [16] | Steckling FM, Farinha JB, Figueiredo FC, Santos D.L, Bresciani G, Kretzmann NA, et al. High-intensity interval training improves inflammatory and adipokine profiles in postmenopausal women with metabolic syndrome. Archives of physiology and biochemistry. 2019; 125(1): 85-91. https://doi.org/10.1080/13813455.2018.1437750. |
| [17] | Cho WJ, Won YD, Moon HH. The effect of combined exercise program on inflammation factors in obese middle-aged women. J Sport Leisure Studies. 2009; 37: 1033–1044. |
| [18] | Asle MZ, Kargarfard M, Marandi SM, et al. Diets along with interval training regimes improves inflammatory & anti-inflammatory condition in obesity with type 2 diabetes subjects. J Diabetes Metab Disord. 2018; 17: 253–267. https://doi.org/10.1007/s40200-018- 0368-0. |
| [19] | Koh Y, Park KS. Responses of inflammatory cytokines following moderate intensity walking exercise in overweight or obese individuals. J Exerc Rehabil. 2017; 13(4), 472-476. doi: 10.12965/jer.1735066.533. |
| [20] | Arsenault BJ, Côté M, Cartier A, Lemieux I, Després JP, Ross R et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis, 2009; 207(2): 530-533. doi: 10.1016/j.atherosclerosis.2009.05.009. |
| [21] | Nunes PR, Martins FM, Souza AP, Carneiro MA, Orsatti CL. Michelin MA, et al. Effect of high-intensity interval training on body composition and inflammatory markers in obese postmenopausal women: a randomized controlled trial. Menopause. 2019; 26(3): 256-264. doi: 10.1097/gme.0000000000001207. |
| [22] | Plotnikoff, R., Eves, N., Jung, M. et al. Multicomponent, home-based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. Int J Obes. 2010; 34: 1733– 1741. https://doi.org/10.1038/ijo.2010.109. |
| [23] | Wedell-Neergaard AS , Lehrskov LL, Christensen RH, Legaard GE, Dorph E, Larsen MK, et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metabolism. 2019; 29(4): 844-855. e3. https://doi.org/10.1016/j.cmet.2018.12.007. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML