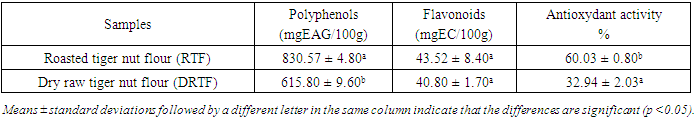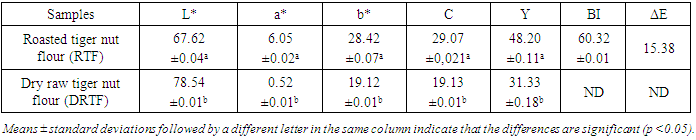-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2022; 12(1): 26-35
doi:10.5923/j.food.20221201.03
Received: Aug. 19, 2022; Accepted: Sep. 5, 2022; Published: Sep. 15, 2022

Investigation of Physicochemical and Biochemical Properties of Roasted Tiger Nut (Cyperus esculentus) Flour
Bou Ndiaye1, 2, Seyni Ndiaye1, 2, Papa Guedel Faye1, 2, Ifeoma C. Orabueze3, Abdou Diouf4, 5, Oumar Ibn Khatab Cisse1, 2, 6, Edouard Mbarick Ndiaye1, 2, Alioune Sow1, 2, 7, Mama Sakho1, 2, Nicolas C. M. Asseyou1, 2
1Laboratoire Eau, Energie, Environnement et Procédés Industriels (LE3PI), Ecole Supérieure Polytechnique / Université Cheikh Anta Diop, Dakar, Sénégal
2Center for Studies on Food Safety and Functional Molecules (CESAM-RESCIF) ESP-UCAD, Dakar, Senegal
3Dept. of Pharmacognosy Faculty of Pharmacy/University of Lagos, Medical campus, Idi-Araba, Nigeria
4Faculté des Sciences et Techniques, Université Cheikh Anta Diop de Dakar, Fann, Sénégal
5Institut de Technologie Alimentaire (ITA) Route des pères Maristes, Hann, Dakar, Sénégal
6Ecole Nationale Superieure d’Agriculture (ENSA), UIDT, Thies, Senegal
7UFR des Sciences Agronomiques, de l’Aquaculture et des Technologies Alimentaires (S2ATA) Université Gaston Berger de Saint Louis, Sénégal
Correspondence to: Bou Ndiaye, Laboratoire Eau, Energie, Environnement et Procédés Industriels (LE3PI), Ecole Supérieure Polytechnique / Université Cheikh Anta Diop, Dakar, Sénégal.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

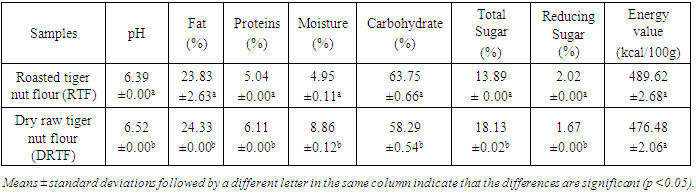
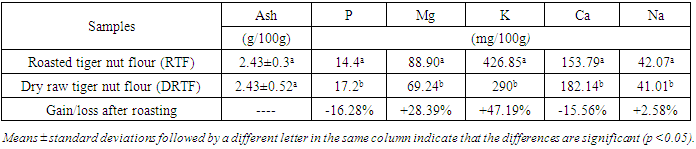
Roasting is a thermal process that has long been used in food processing. This study evaluates the effect of roasting on tiger nuts tuber’s physicochemical and biochemical parameters in order to produce high nutritional quality flour. The raw tiger nut were subjected to roasting procedure. The roasting was done using oven at a temperature of 147°C during 38 min. The nutritional properties analyses were performed on both dry raw tiger nut flour (DRTF) and roasted tiger nut flour (RTF). The data obtained for the analysis revealed that, moisture, total sugar, fat, protein contents, pH and L* index color decrease after roasting process. The contents decrease from 8.86 to 4.95% for moisture, 16.52 to 13,2% for total sugars, 6.11 to 5.04% for proteins, 24.33 for 23.83% for lipids. The pH and L index decrease respectively from 6.52 to 6.39 and 78.54 to 67.62. However, a significant increasing was constated in some parameters after roasting. Contents increase from 1.67 to 2.02% for reducing sugars, 58.29 to 63.76% for carbohydrate, 599.59 to 726.01 mg/100g for mineral elements (P, Mg, K, Ca, Na), 615.80 to 830.57 mgEAG/100g for total phenolic compounds and from 40.8 to 43.52 mgCE/100g for flavonoids. For antioxidant activity, inhibition varies from 32.94 to 60.03%, the energy value from 476.48 to 482.62 Kcal/100g, yellowing index (Y) from 31.33 to 48.20 after roasting. The browning index value were estimated from to 60.32 in roasted tiger nut flour. The infrared spectrum analysis reveals some functional groups and particular bonds in the two flours, and new bands appearing only in the roasted tiger nut flour (RTF). The roasting process reveals significant effects on color, water content, mineral elements, total sugar, polyphenols and antioxidant proprieties. It can be applied to improve the tiger nuts' nutritional value.
Keywords: Tiger nut flour, Roasting, Nutritional value
Cite this paper: Bou Ndiaye, Seyni Ndiaye, Papa Guedel Faye, Ifeoma C. Orabueze, Abdou Diouf, Oumar Ibn Khatab Cisse, Edouard Mbarick Ndiaye, Alioune Sow, Mama Sakho, Nicolas C. M. Asseyou, Investigation of Physicochemical and Biochemical Properties of Roasted Tiger Nut (Cyperus esculentus) Flour, International Journal of Food Science and Nutrition Engineering, Vol. 12 No. 1, 2022, pp. 26-35. doi: 10.5923/j.food.20221201.03.
Article Outline
1. Introduction
- The last decades have been marked by a great interest in food science research and new functional foods development to fight against malnutrition and undernourishment [1], [2]. In this sense, the processing method of local products could play an important role. Roasting is an ancient and a good processing method for preservation, inter-conversion, improving on taste and flavors used y man to conserve his food [3]. In the food industry, roasting is one of the most widely used processes to improve quality and increase stability. Roasting allows the development of desirable aroma, color, and texture and increases palatability [4]. It plays an important role in improving the nutritional value of food. It reduces antinutrient compounds such as phytates, oxalates, tannins, hydrogen cyanide, cyanogenic, and glycosides [5], [6], [7], [8]. It can also contribute to the increase of bioactive compounds such as polyphenols, flavonoids, antioxidant activity, and mineral elements [9]. It also increases the stability of food materials by the destruction of contained microorganisms and toxins. Tiger nut (Cyperus esculentus) is an underutilized tuber of the family Cyperaceae, which produces tuber. Currently, the local use of tiger nuts is limited to casual snacks, similar to peanut. Tiger nut tubers constitute an important source of phytochemicals compounds such as isoflavones, flavonoids, terpenoids, alkaloids and saponins [10] [11]. It’s transformation into new products precooked through roasting or any other process could boost its economic and commercial values and improve the nutritional status of consumers.This present study focused on improving the nutritional value of tiger nuts used for infant food enrichment. Thus, this study sought to investigate the effect of roasting on the nutritional value of tiger nuts at optimal roasting process and conditions [12],[13] obtained were applied in this study.
2. Material and Methods
2.1. Material
- Tiger nuts tuber was purchased from the local market of Bamako. The nuts were cleaned, washed, drained, dried at 37° during 48h in an oven (Mermmert, Germany) and packed in plastic bag until use.
2.2. Methods
2.2.1. Roasting Method
- Dried nuts were roasted according to the optimal roasting process which is 147°C for 38 minutes using an oven (Mermmert, Germany) [12], [13].
2.2.2. Analytical Methods
2.2.2.1. Proximate Composition Analysis of Tiger Nut Flour
- Moisture, crude fat and crude protein and total ash contents of the samples were determined by AOAC methods [14], [15]. Mineral contents were determined by ionic chromatography and expressed as mg mineral/100 g dry weight (dw) sample. Total and reducing sugar was obtained using Luff-Schrool's method [16]. Carbohydrate was determined by difference, and energy content was determined using the Atwater factor (carbohydrate and protein values were each multiplied by 4 Kcal/g, whereas fat values were each multiplied by 9 Kcal/g).
2.2.2.2. Phenolics and Flavonoids Contents
- Polyphenol content was determined using Folin-Ciocalteu reagent according to the method described by Georgé et al. [17] with some modification. Tiger nut flour (2g) sample was soaked in 10 mL of methanol-water 70% (v/v) and mixed for 30 min, and centrifuged at 4000 rpm for 10 min. An aliquot (50 μL) of supernatant and 450 μL of water were mixed and oxidized with 2.5 mL of Folin-Ciocalteu's reagent at 1/10 dilution. The mixture was re-mixed and incubated for 2 min. and neutralized by 2.5 mL of sodium carbonate (75g/L). The mixture was vortexed and placed for 30 min in a water bath at 50°C. After cooling, the absorbance was measured at 760 nm using spectrophotometer (GENESYS 10S UV-VIS, Thermo Scientific). Calibration curve was drawn using the standard drug, gallic acid. The polyphenol content was determined using the calibration curve and expressed as milligrams of gallic acid equivalent (GAE) per gram of the plant extract. Total flavonoids content was determined using the colorimetric method described by Kim et al. [18]. Tiger nut flour (0.5 g) was treated with 10 mL of methanol, stirred and centrifuged. Distilled water (400 μL) and 5% sodium nitrate (30 μL) were added to 2 mL of supernatant. The mixture was mixed and incubated at room temperature for 5 min, after which 20 μL of 10% AlCl3 and 200 μL Na2CO3 were added and the mixture was mixed and re-incubated for another 5 min. After the incubation 250 μL of distilled water were added to the final mixture, and absorbance was determined at 510 nm against a blank (distilled water). The flavonoids content was calculated and expressed as g of catechin equivalent (CE) per 100 g of flour. All determinations were carried out in triplicates.
2.2.2.3. DPPH-Radical Scavenging Activity Assay
- The free-radical-scavenging activity of tiger nut flour extract was measured using 1,1-diphenyl-2-picrylhydrazyl (DPPH) according to Brand-Williams method [19] by soaking 2 g of flour in 20 mL methanol (95%). A measure of 0,1 ml of resultant solution was shaken in 2,9 mL of DPPH (0,6 10-5 mol/L). This was allowed to incubate at room temperature for 30 min in a dark place. The intensity of decolorization (absorbance) of purple free radical DPPH solution was measured at 515 nm using spectrophotometer (GENESYS 10S UV-VIS, Thermo Scientific). The same procedure was repeated without the tiger nut flour extract for control. Thus, to determine the absorbance at t = 0 min, absorbance was immediately taken after adding 0.1 mL methanol to 2.9 mL of DPPH• solution. Methanol solution was used as the blank. The antioxidant activity was calculated using the following equation:
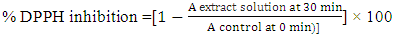 | (1) |
2.2.2.4. pH Determination
- The method of Tortoe and et al. [20] was used for titratable acidity and pH determination with some modification. Tiger nut flour (10g) was mixed with 90 mL of distilled water. The mixture was shaken until particles were evenly suspended and free of lumps and digested for 30 minutes with frequent shaking. The mixture was allowed to stand for 10 mins for the particles to settle. The supernatant was decanted into the 250 mL beaker, and the pH was determined using a pH-meter (Hanna, HI 2211, Romania).
2.2.2.5. Browning Index
- The color of the flour was measured with a chromameter (Minolta CR-310 Osaka, Japan). The color index (browning index IB and yellow index) was determined by the L*a*b system.
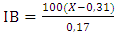 | (2) |
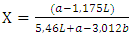 | (3) |
2.2.2.6. Mesure of the Absorbance of Maillard Reaction Product
- Maillard reaction absorbance was analyzed by reading the absorbance at 420 nm, according to the method of Hendel et al. [21]. The measurements were made on an extract obtained by mixing 0.25 g of tiger nut flour with 10 ml of distilled water for 10 min. The suspension was stirred for 15 minutes using a mechanical stirrer (Heidolph MR Hei-Standard, Germany) and centrifuged at 5000 rpm (Universal 16A, D-78532 Tuttlingen, Germany). The supernatants were recovered, and the absorbances at 420 nm (A420) were obtained with a Spectrophotometer (GENESYS 10S UV-VIS, Thermo Scientific).
2.2.2.7. Infra Red Analysis
- The acquisition of infrared spectra of the flour samples is performed by using a branded infrared spectrophotometer (Perkin Elmer LAMDA TWO). The FT-IR spectra were recorded from 4000 to 400 cm-1.
2.2.3. Statistical Analysis
- The data obtained were analysed statistically using the STATISTICA 7.1 software. Differences were considered statistically differently at P< 0.05.
3. Results and Discussion
- The roasted tiger nut flour (RTF) and dry raw tiger nut flour (DRTF) samples were analyzed for physicochemical properties. The proximate profiles of the samples are presented in Tables 1, 2 and 3. The water contents in dry raw tiger nut flour (DRTF) and roasted tiger nut flour (RTF) were 8.86 and 4.95% respectively. The observed low water content in the RTF resulted from the lost of water during roasting. The water may have been lost by evaporation and hydrolysis reactions during roasting. Low moisture content enhances the storage stability of flours. High moisture content encourages biochemical reactions that would lead to food spoilage and microbial growth.The fat contents were 23.83 and 24.33%, respectively, for RTF and DRTF (Table 1). A slight decrease in the percentage of fat was noted. Analysis of variance showed that the difference was significant. This is in contrast to several other studies which have reported an increase in the fat content in several vegetable matrices after roasting [22], [23], [24]. The decrease in fat content during roasting may be due to the destruction of fat.
|
|
|
|
 | Figure 1. Raw tiger nut flour (left side) and roasted tiger nut flour (right side) |
|
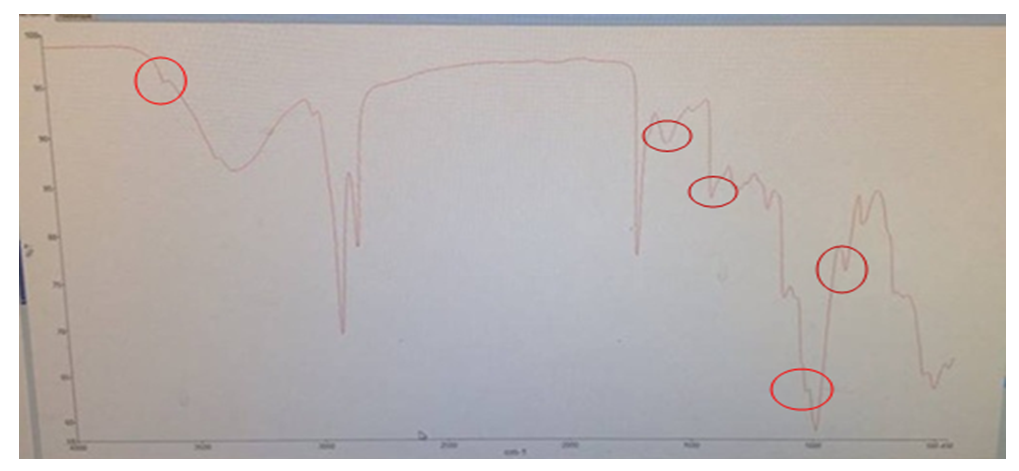 | Figure 2. Infra-red spectrophotometry curve of roasted tiger nut flour (RTF) |
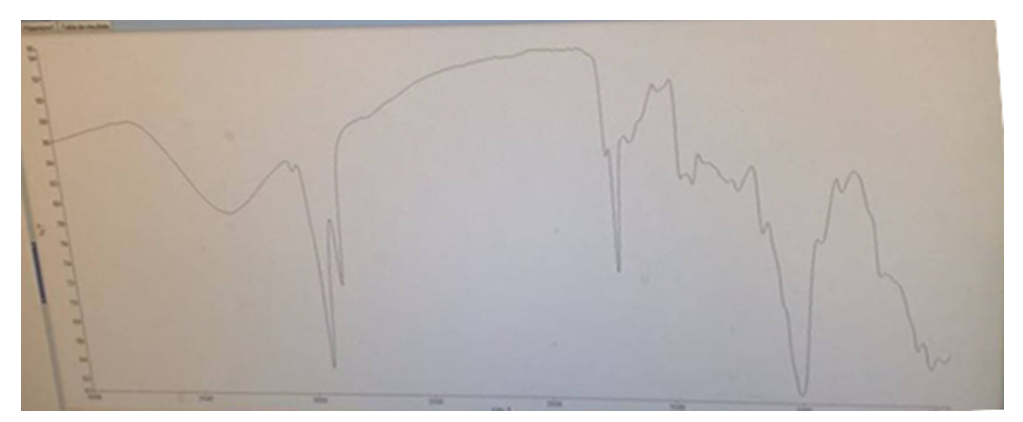 | Figure 3. Infra-red spectrophotometry curve of dry raw tiger nut flour (DRTF) |
4. Conclusions
- The comparative study between raw tiger nut tuber flour and flour from roasted tiger nut tubers showed that the parameters studied were affected by roasting. The contents of water, sugars, polyphenols, flavonoids, and color were significantly positively affected. The infrared spectra reveal the presence of new molecules that could be of pyrazine nature. This flour from roasted tubers could be used to produce food supplements by improving the nutritional value. The flour obtained from roasted nut tubers may well constitute an alternative to cereals, given its composition in phenolic compounds and its high antioxidant capacity. Additional analysis was carried out on the optimal product to extend the understanding.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML