| [1] | Abeshu, Y., Kefale, B. (2017). Effect of some traditional processing methods on nutritional composition and alkaloid content of lupin bean. International Journal of Bioorganic Chemistry, 2(4), 174-179. |
| [2] | Abraham, E. M., Ganopoulos, I., Madesis, P., Mavromatis, A., Mylona, P., Nianiou-Obeidat, I.,... Vlachostergios, D. (2019). The use of lupin as a source of protein in animal feeding: Genomic tools and breeding approaches. International journal of molecular sciences, 20(4), 851. |
| [3] | Agarwal, S., Beausire, R. L., Patel, S., Patel, H. (2015). Innovative uses of milk protein concentrates in product development. Journal of food science, 80(S1), A23-A29. |
| [4] | Akharume, F. U., Aluko, R. E., Adedeji, A. A. (2021). Modification of plant proteins for improved functionality: A review. Comprehensive Reviews in Food Science and Food Safety, 20(1), 198-224. |
| [5] | Aletor, O. (2012). Protein quality evaluation and in vitro multi-enzyme digestibility of some plant protein isolates and concentrates. Archiva Zootechnica, 15(4), 5-16. |
| [6] | Alonso-Miravalles, L., Jeske, S., Bez, J., Detzel, A., Busch, M., Krueger, M.,... Arendt, E. K. (2019). Membrane filtration and isoelectric precipitation technological approaches for the preparation of novel, functional and sustainable protein isolate from lentils. European Food Research and Technology, 245(9), 1855-1869. |
| [7] | Alwan Hamad, A. S., Mohammad, A. M., Esmaeel, H. I., Abdullah, M. K. (2019). Study of the Biological Effect of Substitution Soybean Meal with Lupinus albus L. on Some Somatic and Sex Hormones in Awassi Lambs from Weaning to Puberty. Indian Journal of Public Health Research & Development, 10(8). 1992-1995. |
| [8] | A.O.A.C, 2016, “fficial methods of analysis of AOAC international 20th(ed) current through revision 2. ATKINSON, M. A. & FURTH, S. L. 2011. Anemia in children with chronic kidney disease,” Nat. Rev. Nephrol., 7:. 635. |
| [9] | Bader, S., Oviedo, J. P., Pickardt, C., Eisner, P. (2011). Influence of different organic solvents on the functional and sensory properties of lupin (Lupinus angustifolius L.) proteins. LWT-Food Science and Technology, 44(6), 1396-1404. |
| [10] | Banti, M., Bajo, W. (2020). Review on Nutritional Importance and Anti-nutritional Factors of Legumes. International Journal of Nutrition and Food Sciences, 9(6), 138. |
| [11] | Bartham, D.,1972 “97:142.,” Analyst,. 97, 142. |
| [12] | Bartkiene, E., Juodeikiene, G., Vidmantiene, D. (2012). Nutritional quality of fermented defatted soya and flaxseed flours and their effect on texture and sensory characteristics of wheat sourdough bread. International journal of food sciences and nutrition, 63(6), 722-729. |
| [13] | Bartles, H., Bohmer, M., Heirli, C. (1972). Colorimetric kinetic method for creatinine determination in serum and urine. Clin Chem Acta, 37, 193-195. |
| [14] | Bender, A. E., Doell, B. H. (1957). Biological evaluation of proteins: a new aspect. British Journal of Nutrition, 11(2), 140-148. |
| [15] | Bergmeyer, H. U., Harder, M. (1986). A colorimetric method of the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Clin. Biochem, 24(48), 1-488. |
| [16] | Burger, T. G., Zhang, Y. (2019). Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends in Food Science & Technology, 86, 25-33. |
| [17] | Chew, P. G., Casey, A. J., Johnson, S. K. (2003). Protein quality and physico-functionality of Australian sweet lupin (Lupinus angustifolius cv. Gungurru) protein concentrates prepared by isoelectric precipitation or ultrafiltration. Food chemistry, 83(4), 575-583. |
| [18] | da Silva, M. E. T., de Paula Correa, K., Martins, M. A., da Matta, S. L. P., Martino, H. S. D., & dos Reis Coimbra, J. S. (2020). Food safety, hypolipidemic and hypoglycemic activities, and in vivo protein quality of microalga Scenedesmus obliquus in Wistar rats. Journal of Functional Foods, 65, 103711. |
| [19] | Doumas, B., (1971) “Standard methods of clinical chemistry.,” Acad. Press. New York, 4,. 85. |
| [20] | Drulyte, D., Orlien, V. (2019). The effect of processing on digestion of legume proteins. Foods, 8(6), 224. |
| [21] | Erbaş, M., Certel, M., Uslu, M. K. (2005). Some chemical properties of white lupin seeds (Lupinus albus L.). Food chemistry, 89(3), 341-345. |
| [22] | Facciolongo, A. M., Rubino, G., Zarrilli, A., Vicenti, A., Ragni, M., Toteda, F. (2014). Alternative protein sources in lamb feeding 1. Effects on productive performances, carcass characteristics and energy and protein metabolism. Progr. Nutr, 16, 105-115. |
| [23] | FAO/WHO, 1991“Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation Paper 51. Rome: FAO.,” FAO Food Nutr., p. Paper 51. Rome: FAO. |
| [24] | Feyzi, S., Varidi, M., Zare, F., & Varidi, M. J. (2017). A comparison of chemical, structural and functional properties of fenugreek (Trigonella foenum graecum) protein isolates produced using different defatting solvents. International journal of biological macromolecules, 105, 27-35. |
| [25] | Glencross, B., Sweetingham, M., Hawkins, W. (2010). A digestibility assessment of pearl lupin (Lupinus mutabilis) meals and protein concentrates when fed to rainbow trout (Oncorhynchus mykiss). Aquaculture, 303(1-4), 59-64. |
| [26] | Griffiths, M. R., Strobel, B. W., Hama, J. R., Cedergreen, N. (2021). Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environmental Sciences Europe, 33(1), 1-12. |
| [27] | Gulewicz, P., Martínez-Villaluenga, C., Frias, J., Ciesiołka, D., Gulewicz, K., & Vidal-Valverde, C. (2008). Effect of germination on the protein fraction composition of different lupin seeds. Food Chemistry, 107(2), 830-844. |
| [28] | Hassan, A. A., Rasmy, N. M., El-Gharably, A., Abd El-Megied, A. A., Gadalla, S. M. (2014). Hypocholesterolemic effects of soybean and sweet lupine tempeh in hypercholesterolemic rats. International Journal of Fermented Foods, 3(1), 11-43. |
| [29] | Hegsted, D. M. (1977). Protein quality and its determination. Food proteins. |
| [30] | Henderson, J. W., Ricker, R. D., Bidlingmeyer, B. A., Woodward, C. (2000). Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Amino acid analysis using Zorbax Eclipse-AAA columns and the Agilent, 1100, 1-10. |
| [31] | Iqbal, A., Khalil, I. A., Ateeq, N., & Khan, M. S. (2006). Nutritional quality of important food legumes. Food chemistry, 97(2), 331-335. |
| [32] | Janusz, P. (2017). White lupin (Lupinus albus L.)–nutritional and health values in human nutrition–a review. Czech Journal of Food Sciences, 35(2), 95-105. |
| [33] | Jayasena, V., Chih, H. J., Nasar-Abbas, S. (2010). Functional properties of sweet lupin protein isolated and tested at various pH levels. Research Journal of Agriculture and Biological Sciences, 6(2), 130-137. |
| [34] | Jezierny, D., Mosenthin, R., Bauer, E. (2010). The use of grain legumes as a protein source in pig nutrition: A review. Animal Feed Science and Technology, 157(3-4), 111-128. |
| [35] | Kar, M., Chourasiya, Y., Maheshwari, R., Tekade, R. K. (2019). Current Developments in Excipient Science: Implication of Quantitative Selection of Each Excipient in Product Development. In Basic Fundamentals of Drug Delivery (pp. 29-83). Academic Press. |
| [36] | Khan, M. K., Karnpanit, W., Nasar-Abbas, S. M., Huma, Z. E., Jayasena, V. (2015). Phytochemical composition and bioactivities of lupin: a review. International journal of food science & technology, 50(9), 2004-2012. |
| [37] | Lafarga, T., Villaró, S., Bobo, G., Aguiló-Aguayo, I. (2019). Optimisation of the pH and boiling conditions needed to obtain improved foaming and emulsifying properties of chickpea aquafaba using a response surface methodology. International Journal of Gastronomy and Food Science, 18, 100-177. |
| [38] | Lestingi, A., Facciolongo, A. M., Jambrenghi, A. C., Ragni, M., Toteda, F. (2016). The use of peas and sweet lupin seeds alone or in association for fattening lambs: Effects on performance, blood parameters and meat quality. Small Ruminant Research, 143, 15-23. |
| [39] | Lopez, M. J., Mohiuddin, S. S. (2021). Biochemistry, Essential Amino Acids. StatPearls [Internet]. |
| [40] | Lukuyu, B., Okike, I., Duncan, A. J., Beveridge, M., Blummel, M. (2014). Use of cassava in livestock and aquaculture feeding programs (Vol. 25). ILRI (aka ILCA and ILRAD). |
| [41] | Martínez-Villaluenga, C., Urbano, G., Porres, J. M., Frias, J., Vidal-Valverde, C. (2007). Improvement in food intake and nutritive utilization of protein from Lupinus albus var. multolupa protein isolates supplemented with ascorbic acid. Food Chemistry, 103(3), 944-951. |
| [42] | Mendes, F. Q., de Almeida Oliveira, M. G., Cardoso, L. R., Costa, N. M. B., SantAna, R. D. C. O. (2007). Digestibilidade protéica e caracterização bromatológica de linhagens de soja com ausência ou presença do inibidor de tripsina kunitz e das isozimas lipoxigenases. Bioscience Journal, 23(1) 14-21. |
| [43] | Monteiro, M. R. P., Costa, A. B. P., Campos, S. F., Silva, M. R., da Silva, C. O., Martino, H. S. D., & Silvestre, M. P. C. (2014). Evaluation of the chemical composition, protein quality and digestibility of lupin (Lupinus albus and Lupinus angustifolius). Mundo da Saúde, São Paulo, 38, 251-259. |
| [44] | Paredes-López, O., Cervantes-Ceja, M. L., Vigna-Pérez, M., Hernández-Pérez, T. (2010). Berries: improving human health and healthy aging, and promoting quality life—a review. Plant foods for human nutrition, 65(3), 299-308. |
| [45] | Phillips, D. E., Eyre, M. D., Thompson, A., Boulter, D. (1981). Protein quality in seed meals of Phaseolus vulgaris and heat-stable factors affecting the utilisation of protein. Journal of the Science of Food and Agriculture, 32(5), 423-432 |
| [46] | Piornos, J. A., Burgos-Díaz, C., Ogura, T., Morales, E., Rubilar, M., Maureira-Butler, I., Salvo-Garrido, H. (2015). Functional and physicochemical properties of a protein isolate from AluProt-CGNA: A novel protein-rich lupin variety (Lupinus luteus). Food Research International, 76, 719-724. |
| [47] | Rodríguez-Ambriz, S. L., Martínez-Ayala, A. L., Millán, F., Davila-Ortiz, G. (2005). Composition and functional properties of Lupinus campestris protein isolates. Plant Foods for Human Nutrition, 60(3), 99-107. |
| [48] | Šćiban, M., Vasić, M., Prodanović, J., Kukić, D., Vasić, V., Omorjan, R. and Antov, M., 2021. Water turbidity removal by faba bean (Vicia faba) in relation to composition of aqueous extract of seed. International Journal of Environmental Science and Technology, 18, 2847–2854. |
| [49] | Shermer, G., Jones, K.,1976. Public Housing is the Tenants. |
| [50] | Sobotka, W., Stanek, M., Bogusz, J. (2016). Evaluation of the nutritional value of yellow (Lupinus luteus) and blue lupine (Lupinus angustifolius) cultivars as protein sources in rats. Annals of Animal Science, 16(1), 197. |
| [51] | Stanek, M., Rotkiewicz, T., Sobotka, W., Bogusz, J., Otrocka-Domagała, I., Rotkiewicz, A. (2015). The effect of alkaloids present in blue lupine (Lupinus angustifolius) seeds on the growth rate, selected biochemical blood indicators and histopathological changes in the liver of rats. Acta Veterinaria Brno, 84(1), 55-62. |
| [52] | Straková, E., Všetičková, L., Kutlvašr, M., Timová, I. and Suchý, P., 2021. Beneficial effects of substituting soybean meal for white lupin (Lupinus albus, cv. Zulika) meal on the biochemical blood parameters of laying hens. Italian Journal of Animal Science, 20(1),.352-358. |
| [53] | Starkute, V., Bartkiene, E., Bartkevics, V., Rusko, J., Zadeike, D., Juodeikiene, G. (2016). Amino acids profile and antioxidant activity of different Lupinus angustifolius seeds after solid state and submerged fermentations. Journal of food science and technology, 53(12), 4141-4148. |
| [54] | Sun, J., Zhou, W., Huang, D., Yan, L. (2018). 3D food printing: Perspectives. In Polymers for food applications. 725-755). Springer, Cham. |
| [55] | Taglieri, I., Macaluso, M., Bianchi, A., Sanmartin, C., Quartacci, M. F., Zinnai, A., Venturi, F. (2021). Overcoming bread quality decay concerns: Main issues for bread shelf life as a function of biological leavening agents and different extra ingredients used in formulation. A review. Journal of the Science of Food and Agriculture, 101(5), 1732-1743. |
| [56] | Thakur, A., Sharma, V., Thakur, A. (2019). An overview of anti-nutritional factors in food. Int. J. Chem. Stud, 7(1), 2472-2479. |
| [57] | Terrell, J.F (1992) “Analysis of complex mixtures of amino acids using the HP 1050 modular HPLC.,” Appl. note, 212–228. |
| [58] | Yagi, S. M., & Yagi, A. I. (2018). Traditional medicinal plants used for the treatment of diabetes in the Sudan: a review. African Journal of Pharmacy and Pharmacology, 12(3), 27-40.. |
| [59] | Viveros, A., Centeno, C., Arija, I., Brenes, A. (2007). Cholesterol-lowering effects of dietary lupin (Lupinus albus var Multolupa) in chicken diets. Poultry science, 86(12), 2631-2638. |
| [60] | Vogelsang-O’Dwyer, M., Bez, J., Petersen, I. L., Joehnke, M. S., Detzel, A., Busch, M.,... Zannini, E. (2020). Techno-functional, nutritional and environmental performance of protein isolates from blue lupin and white lupin. Foods, 9(2), 230. |
| [61] | Zheng, H. G., Yang, X. Q., Tang, C. H., Li, L., Ahmad, I. (2008). Preparation of soluble soybean protein aggregates (SSPA) from insoluble soybean protein concentrates (SPC) and its functional properties. Food research international, 41(2), 154-164. |


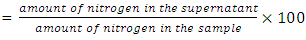
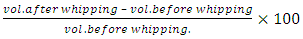
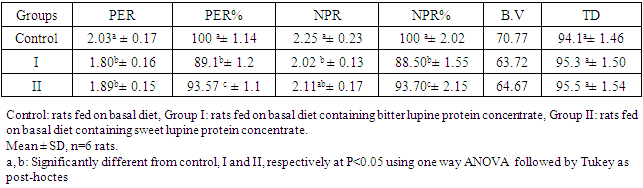
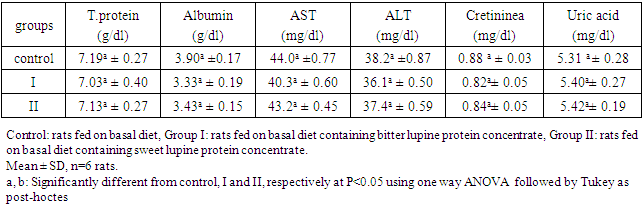
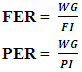 Feed Efficiency Ratio (FER), Protein Efficiency Ratio (PER), weight gain (WG), food Intake (FI), protein intake (PI) (Hegsted, 1997 and Martínez et al., 2007).Net protein Ratio (NPR) was determined in the 14th day of the experiment, weight gain of the test group plus the weight loss of the group fed on low protein diet, regarding the protein intake of the test group by the method of (Bender and Doell 1957; Martínez et al., 2007). The relative of protein efficiency ratio (PER-R) and Net protein Ratio (NPR-R) were determined to be 100% of the PER and NPR results of the basal diet (Aletor, 2012).
Feed Efficiency Ratio (FER), Protein Efficiency Ratio (PER), weight gain (WG), food Intake (FI), protein intake (PI) (Hegsted, 1997 and Martínez et al., 2007).Net protein Ratio (NPR) was determined in the 14th day of the experiment, weight gain of the test group plus the weight loss of the group fed on low protein diet, regarding the protein intake of the test group by the method of (Bender and Doell 1957; Martínez et al., 2007). The relative of protein efficiency ratio (PER-R) and Net protein Ratio (NPR-R) were determined to be 100% of the PER and NPR results of the basal diet (Aletor, 2012).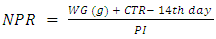 True digestibility was also determined as follow:
True digestibility was also determined as follow: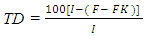 True digestibility (TD), the amount of nitrogen intake (I), the amount of nitrogen excreted in feces (F), the nitrogen fecal loss of the low protein diet group (FK) (Phillips, et al., 1981). At the end of experiment blood samples were taken from the orbital plexus of eye of each rat (Shermer and Jones, 1967) serum was separated and its biochemical parameters were determined.
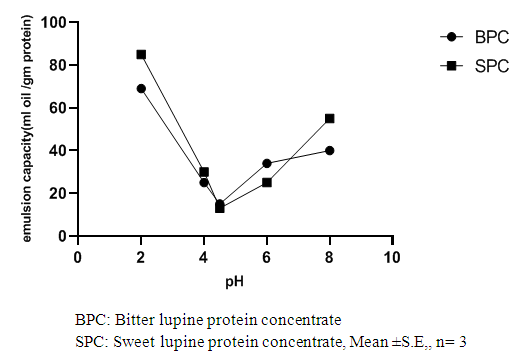
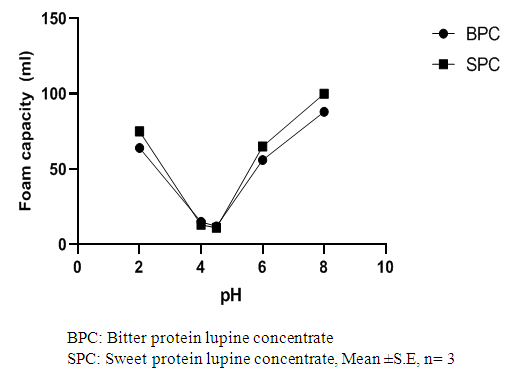
True digestibility (TD), the amount of nitrogen intake (I), the amount of nitrogen excreted in feces (F), the nitrogen fecal loss of the low protein diet group (FK) (Phillips, et al., 1981). At the end of experiment blood samples were taken from the orbital plexus of eye of each rat (Shermer and Jones, 1967) serum was separated and its biochemical parameters were determined.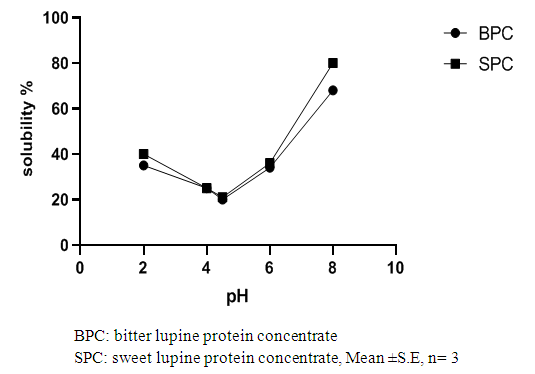
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML