-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2021; 11(2): 52-64
doi:10.5923/j.food.20211102.03
Received: Sep. 1, 2021; Accepted: Sep. 22, 2021; Published: Dec. 27, 2021

Improvement of Injera Shelf Life Through the Use of Silver (Ag) and Zinc Oxide (ZnO) Nano Particles
Oli Legassa1, Ashagrie Zewdu2, Eyobel Mulugeta3
1Food Science and Nutrition Directorate, Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia
2School of Food Science and Nutrition, Addis Ababa University, Addis Ababa, Ethiopia
3Ministry of Innovation and Technology, Ethiopian Biotechnology Institute, Emerging Technology Center, Nanotechnology Directorate, Addis Ababa, Ethiopia
Correspondence to: Oli Legassa, Food Science and Nutrition Directorate, Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

About two-third of Ethiopian diet consists of Injera which is prepared from the flour of tef (Eragrostis tef (Zucc) Trotter), water and starter. Even though it is a nutritious food, the shelf life of Injera does not exceed 3 days. The aim of this study was to evaluate the effect of green synthesized silver (AgNPs) and zinc oxide (ZnONPs) nano particles in improving Injera shelf life. Ag and ZnONPS were synthesized from Eucalyptus globulus and Calpurnia aurea (Ait.) Benth extracts, respectively. Formation of nano particles were confirmed by characterization techniques (ultraviolet-visible spectroscopy, scanning electron microscopy and X-ray diffraction). Antifungal test was conducted using disc diffusion method. Both nano particles were coated on the interior and external surface of packaging materials by dip-coated method and Injera was packed in the plastic bags. The shelf life was determined as one day before mold growth appearance. Moisture content and pH was determined by oven and pH meter, respectively. Pour plate method was used for mold and yeast count. Finally, migration level was determined by microwave plasma - atomic emission spectroscopy. The results from UV-vis spectroscopy showed that characteristic peaks observed at 420 and 300 to 400 nm for silver and zinc oxide nano particles, respectively. Silver nano particles were mostly an irregular with few rod shaped whereas that of zinc oxide was mostly rod shaped. Both of them had significant antifungal effect. The shelf life of the stored Injera samples was increased significantly up to 24 days at higher concentrations. Both nano particles have no significant effect on moisture and pH of stored Injera. The colony forming unit per gram of molds and yeast decreased as the coating percentage increased. At 50 % concentration, the migration was 1.34 and 375 mg/Kg for silver and zinc oxide nano particles, respectively. Their migration was relatively high. Because, it needs further study to use them for Injera storage. Thus, it would be advisable to incorporate them as ingredients of plastics to decrease migration problem.
Keywords: Injera, Nano particles, Moulds and yeasts, Shelf life, Packaging, Storage
Cite this paper: Oli Legassa, Ashagrie Zewdu, Eyobel Mulugeta, Improvement of Injera Shelf Life Through the Use of Silver (Ag) and Zinc Oxide (ZnO) Nano Particles, International Journal of Food Science and Nutrition Engineering, Vol. 11 No. 2, 2021, pp. 52-64. doi: 10.5923/j.food.20211102.03.
Article Outline
1. Introduction
- The shelf life of a food is described as the period for which it remains safe and suitable for consumption. The shelf life of baked foods is limited by different factors like water activity and microbiological spoilage [1]. Foods with pH value less than 4.5 are more susceptible to mold spoilage because of their tolerance to acid conditions than bacteria [2]. Injera is one of baked products with pH value less than this value. In addition to this, it has high moisture content that makes it more conducive for mold growth. As a result, shelf life of Injera does not usually exceed 3 days [3]. About two-third of Ethiopian diet [4] consists of Injera, a thin, fermented bread from flour of tef (Eragrostis tef (Zucc) Trotter). The three fungal species found to be responsible for Injera spoilage are Penicillium sp. and Rhizopus sp and Aspergillus niger [3]. Different preservation methods as reduction of available water and use of chemical preservatives are commonly used to elongate shelf life of bakery foods. Chemical preservatives (0.1% of sodium benzoates and benzoic acids) have prolonged the shelf life of Injera for up to 12 days [3]. However, they are applied as additives, which are not convenient for Injera preparation. Reduction of available water also does not work for Injera as its high moisture content is one of Injera quality. Another recently growing food preservation method is the development of nano-enabled food packaging. Silver nano particles (AgNPs) and zinc oxide nano particles (ZnONPs) are metal’s nano particles used in packaging materials as antimicrobial agents [5]. They can be hosted in different polymers [6,7]. Such metal nano particles can be coated or directly incorporated in the synthesis processes of packaging materials [8]. Antimicrobial action may be obtained from the packaging by releasing the biocide directly into the food [9]. This can be exerted by both organic and inorganic materials [10]. The former ones are mostly organic acids and enzymes whereas the latter ones are nano particles of metals or metal oxides. The organic antimicrobial materials are less stable at high temperatures whereas metal and metal oxide nano particles withstand a harsher processing condition [11] which is additional advantage of using inorganic materials.Migration of nano particles from packaging materials to food is reported in some researches. However, European Food Safety Authority and other national food safety authorities regulate the application of AgNPs in food packaging. It provided upper limits of Ag migration to less than 0.05 mg/kg in food [12]. It has also addressed that consumption of ZnONPs should not exceed 250 mg/kg, which is recommended daily intake (RDI) for Zn.Different studies were conducted concerning the migration of silver nano particles from different types of nanocomposites (Low Density Poly Ethylene, 1,1 Diphenylethylene and polypropylene) into food simulants [13]. It was found that the Ag migration is well below the limits stated by the European Union legislation. For example, the research studied the effect of time and temperature on the migration of silver from polyethylene (PE) nanocomposites to boneless chicken breasts and found that migration of silver NPs was in a range from 0.003 to 0.005 mg/L [14]. Also, bread samples had recorded the lowest migration (<0.05 mg/L) level of AgNPs than other food samples [11]. Since Injera is somewhat similar products with bread, this type of antimicrobial packaging can also works for it. Therefore, the aim of this study was to evaluate the effects of green synthesized Ag and ZnONPs in improvement of Injera shelf life.
2. Materials and Methods
2.1. Materials
- Eucalyptus globulus and (Calpurnia aurea (Ait.) Benth.) leaves were used as reducing and caping agent for the green synthesis of nano particles. AgNO3 and Zn(NO3)2.6H2O were used as primary precursors. Mancozeb, a broad spectrum antifugus, was used as control. Chramphenicol was used in potatoes dextros agar (PDA) as bacterial suppressant in pure culture development and antimicrobial tests. Alcohol (70%) was used as disinfectant. For dics preparation, Whattman filter paper (No:1) was cut into pieces. Low density polyethylene (LDPE) polymers (18 x 21 cm zipper bag) was used for preparation of nano-enabled food contact packaging materials.
2.2. Experimental Design
- Completely randomized design (CRD) was used in this study. All the treatments were triplicated. For anti-microbial determination, both AgNPs and ZnONPs were used at 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100% concentrations. Extract +AgNPs (Extr + AgNPs) was also considered as one treatment. Totally, 12 treatments were compared including control sample (Mancozeb, a broad specrum fungicide [15]. For dose determination during shelf life test, both types of nano particles were taken at concentrations of 10, 30, 50, 70, 90, and 100%, whereas uncoated zipper bag was used as control sample. Totally, 7 samples including the control sample were used for the treatments.However, for other parameters (mold and yeast cfu/g, moisture content, pH, and migration test), both treatments (ZnONPs and AgNPs) were used at 10, 50 and 90% concentrations. Extract of Eucalyptus + AgNPs (Ext + AgNPs) and Extract of Calpurnia aurea (Ait.) Benth + ZnONPs (Ext + ZnONPs) were used as treatments. Untreated plastic zipper bag was used as a control. Totally, 5 treatments were analyzed separately including the control sample.
2.3. Plant Extract Preparation
- Fresh and healthy leaves were collected from fields and washed well with pure water. Then, they were air-dried and ground to fine powder. About 100 g of powder and 800 mL of distilled water were allowed to mix in 1000 mL beaker [16] and heated on magnetic stirror. Afterwards, the extract was allowed to cool to room temperature and filtered using Whatman® No.1 filter. The extracts were kept in separate beakers at 4°C [17] for further reaction with AgNO3 and ZnNO3.6H2O, respectively.
2.4. Synthesis, and Characterization of Ag and ZnONano Particles
2.4.1. Synthesis of Ag and ZnO Nano Particles
- The synthesis of both nano particles were carried out in aqueous solutions of silver nitrate (AgNO3) and zinc nitrate Zn(NO3)2. The optimization process was done for both nano particles by dissolving about 0.5094, 0.6113, and 0.7641 g of AgNO3 in 300 ml of deionized water that gives 10, 12, and 15 mM of solution, respectively. For Zn(NO3)2 , about 10, 15, and 20 g was dissolved in 300ml of deionized water that gives 852.3, 1136.4, 14773.2 mM of solution, respectively. The mixture ratio were 1:1, 3:1 and 3:2 for both AgNO3 and Zn(NO3)2 to extract. The mixtures were heated at 82°C for 45 minutes [18] while stirring at 1500 rpm. Based on the developed color, 15 mM at 3:2 and 1136.4 mM at 3:1 precursor solution to extract ratio were selected as optimum for AgNO3 and Zn(NO3)2, respectively.Both solutions were kept at room temperature for 48 h. After settlement, the mixtures were centrifuged at 12,000 rpm for 10 minutes [19]. The supernatant was discarded and the pellets were collected and washed well with distilled water. For silver nano particles, the collected pellets were air-dried and grounded using mortar and pestle. But, the pellets from zinc nitrate solution were oven dried at 105°C for overnight and finally incinerated at 450°C for 3 h [20] in muffle furnace. Both nano particles were stored at 4°C for characterization and further use.
2.4.2. Characterization of the Synthesized Nano Particles
- The samples were investigated by X-ray Diffractometer (XRD) on X’pert High Score powder diffractometer (CuKα X-ray radiation, λ = 0.1541 nm) to study the formation cristalline materials and to estimate the crystallite size of the as-synthesized nano particles. The surface morphology and particle size was investigated by ultra-high-resolution field emission scanning electron microscopy(SEM) (NOVANANO 450, US FEI Corporation). Ultra-violet (UV-vis) spectroscopy (Shimadzu UV-vis 2550) was used to measure the absorption between 200 and 800 nm [21]. The average crystallite size (D) was calculated by the Debye Scherrer formula [20] in X’pert HighScore Plus software (2004 PANalytica B.V.).
2.5. Preparation of Nano-Enabled Coatings from Ag and ZnO Nano Particles
- Dip coating was used to attach the nano particles onto the intireror and external surface of plastic packages. Zipped plastic bag containers (18 cm x 21 cm) were disinfected by 70% ethanol and dried at 60°C in an oven [11] for half an hour. The plastic bags were e dip-coated in different concentration level of (10, 20…, 100%) Ag and ZnO nano particles solutions for 5 min and dried at 60°C in an oven.
2.6. Performance Evaluation
- a) Pure culture preparation: Sterile molten potato dextrose agar (PDA) with chloramphenicol (60 mg/L) was poured into petri dishes and allowed to solidify. Different colonies were taken from Injera stored for 5 days using sterile needle and transferred to plates containing solidified PDA agar. Colonies were selected based on their color (Aspergillus Niger-black, Penicillium spp-green, Rhizopus-dark brown). The plates were incubated at 25°C for 72 h [22]. Growth and similar sub-culturing were perforemed up to fourth generation to obtain pure culture. The fungal isolates were identified by microscope to confirm that the isolated cultures were pure cultures.b) Antifungal evaluation of Ag and ZnONPs: Disc diffusion method was used to assess the antifungal activities of both Ag and ZnONPs as method described by [23]. Whattman filter paper (No:1) was used to prepare discs approximately 6 mm in diameter [16]. The suspension was adjusted to 0.5 mc Farland standard using cytometry method. The antifungal activity was evaluated against the final fungal concentration of 106 cfu/ml [24]. About 1 mL of spore suspension was uniformly spread on sterile Petri plates containing PDA medium.Sterile neutral discs (6 mm in diameter) were impregnated with different concentrations (10, 20, 30, 40, 50, 60, 70, 80, 90, and 100%) of Ag and ZnONPs and aseptically placed on the inoculated culture plates using a sterile forceps. The plates were placed in an incubator at 25°C for 72 h [22]. The antifungal activity was determined by measuring the zone of inhibition (mm) using digital caliper. The disc impregnated with Mancozeb was used as a control.
2.7. Sampling Method
- Sampling was conducted by taking pieces of Injera from every quarter of the Injera roll and blending it. For fungus colony growth and migration evaluation, the stored injera were sampled at 2, 4, 6, 8, and 10 days of storage.
2.8. Determination of pH and Moisture Content of Injera
- The sampling of Injera and pH test were performed as procedure used by [3]. An electronic pH meter was used to measure pH. Each homogenized Injera suspension (10 g of ground Injera added to 100 mL distilled water) was measured. For moisture, about 5 g of the Injera samples were measured and dried at 100°C in an oven until constant weight reached [25]. Moisture content was calculated as:
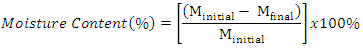
2.9. Shelf life Test
- Shelf life is defined as the period in which visible mould and yeast growth not seen [26]. The stored Injera was monitored daily until mould growths occur. The shelf life was interpreted in relation to controle sample.
2.10. Mould and Yeast
- Pour plate method [22] was used to analyze yeast and mould counts. About 9.75 g of PDA was weighed and dissolved into 250 mL distillled water. The medium was sterilized in an autoclave at 121°C for 15 min and finally chloramphenicol was added aseptically to molten PDA at 45°C. About 10 g of Injera samples was aseptically taken from all quarter parts and added with 90 mL of sterile water and homogenized for 3 min [27] using Vortex. About 1 g of each sample was added into test tubes containing 10 mL sterile water and used as stock solutions. From this, 1 mL was removed and added to another set of test tubes containing 9 mL sterile water which made 10-1 dilution. The same procedure was repeated to make 10-5 dilution. Then, 1 mL of both 10-2 and 10-5 dilutions were added into separate sterile Petri dishes and sterile molten agar was poured into the plates. The inoculated plates were allowed to set and incubated. The plates were kept in an inverted position to avoid water condensation. The PDA plates were incubated at 25°C for 72 h. The number of colonies found on each media was counted. Plates containing 10-150 colonies were counted [28]. It was determined as Numerous To Count (NTC) if above 150, and Few To Count (FTC) if below 10.
2.11. Migration Test (Ag and Zn)
- Daily visual observation was performed on packaged Injera stored in the antimicrobial containers and conventional containers for 10 days [11]. Microwave plasma-atomic emission spectroscopy (MP-AES) was used to determine the amount of Ag and Zn in the sample. The samples were analyzed after acid digestion.
2.12. Statistical Analysis
- A completely randomized design statistics were carried out with the analysis of variance (One-way ANOVA) procedure in SPSS software. Differences among average values were detected by Duncan’s multiple range test (p < 0.05). The result was interpreted as mean ± standard error.
3. Results and Discussion
3.1. Synthesis of Ag and ZnO NPs
- Color change is the simple method of nano particles formation confirmation. Reduction (Ag+ to Ag0) of silver ions into silver nano particles during exposure to plant extracts was observed by the color change from deep red to dark brown. Also the reduction (Zn2+ to Zno) of ZnONPs biosynthesized from Calpurnia aurea (Ait.) Benth leaves extract was confirmed by a color change of deep red to light yellow, indicating formation of ZnONPs. This color change is due to the Surface Plasmon Resonance (SPR) Phenomenon. The metal nano particles have free electrons, which give the SPR absorption band, due to the combined vibration of electrons of metal nano particles in resonance with light wave. It is caused by excitation of surface plasmon vibrations in Ag and ZnO nano particles [29]. As concentration of AgNO3 increased the color of its mixture with leaves extract changed from very slight brown to dark brown whereas that of ZnO(NO2)3 was chanched from dark red to very slight yellow which is indicator for development of nano particles. The UV-vis spectroscopy result is shown in Figure 1 to more confirm development of nano particles.
 | Figure 1. Image of the Synthesised Nanoparticles After Optimization |
3.2. Characterization of Ag and ZnO NPs
- a) UV-vis spectroscopy: The UV-vis spectrum of the synthesized AgNPs and ZnONPs is shown in Fig 2. Synthesis of the AgNPs and ZnONPs was confirmed by recording the absorption spectrum at a wavelength range of 200-800 nm [39]. The characteristic surface plasmon absorption band (SPAB) was observed at 420 nm for AgNPs. There is a report that SPAB of AgNPs from leaf extract was in range of 436 to 446 nm [29]. Other study has also reported that SPAB of AgNPs was observed at 440 nm [16]. Broadening of peak of silver nano particles formed in the reaction indicated that the particles were poly dispersed. It was similar with the study conducted on AgNPs [30]. For ZnONPs, the characteristic surface plasmon absorption band was observed at 300 to 400 nm (Fig 2). It was reported that ZnONPs biosynthesized from different plants have showed characteristic surface plasmon absorption around 400 nm [31]. Another report has also revealed that the sharp band of zinc colloids was observed at 361 nm [32]. There is also reported result that the SPAB of ZnNPs was in range of 299 to 311 nm [17]. In addition to these, other report shows that synthesized zinc oxide nano particles were confirmed by the UV-vis absorption spectra at the wavelength range of 380 to 386 nm [31], which is the characteristic wavelength range of zinc oxide nano particles. The minor difference between these reports and the current result may be raised from plant source difference.The broadening of peaks of ZnONPs from Calpurnia aurea (Ait.) Benth extract was greater than that of AgNPs from Eucalyptus extract which indicates that ZnONPs were smaller. Broadening of peak of zinc oxide nano particles formed in the reaction indicated that the particles are polydispersed. There is study proved that the produced ZnONPs was poly-dispersed which means not aggregated in its nature [17]. It was similar with finding of [33] that conducted on ZnONPs.
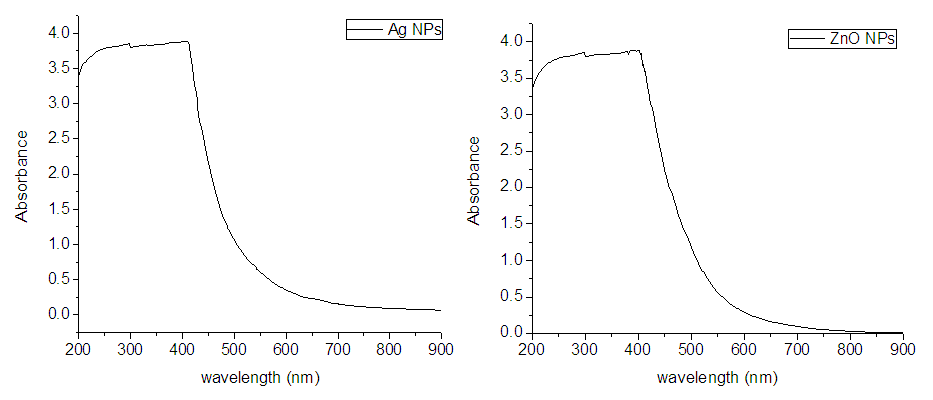 | Figure 2. UV-Vis Spectrum of Ag and ZnONPs at 200-800nm Wavelength |
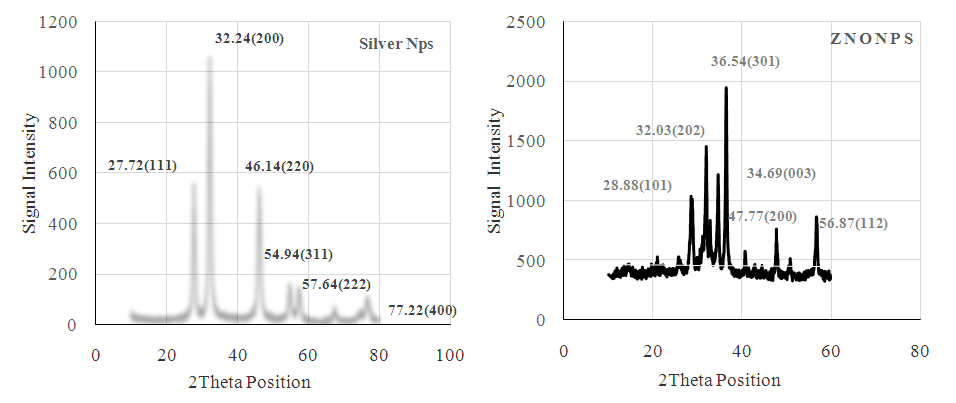 | Figure 3. XRD Spectra of AgNPs and ZnONPs from Eucalyptus Calpurnia aurea (Ait.) Benth, Extract |
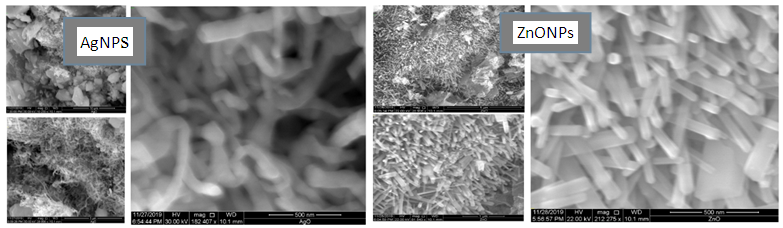 | Figure 4. SEM Image of Biosynthesized AgNPs and ZnONps |
3.3. Antimicrobial Activity of Ag and ZnONPs
- The results on the antimicrobial test of both Ag and ZnONPs at different percentages are shown in Fig 5. It shows the patterns of antimicrobial capacity of Ag and ZnONPs which was determined by applying them against Aspegillus Niger, Penicillum spp and Rhizopus fungal types. Both nano particles (AgNPs and ZnONPs) had significantly (p<0.05) affected the growth of all fungus. The diameter of inhibited zone was increased as the concentration level of Ag and ZnONPs increased. There was a numerical difference between both types of treatments.
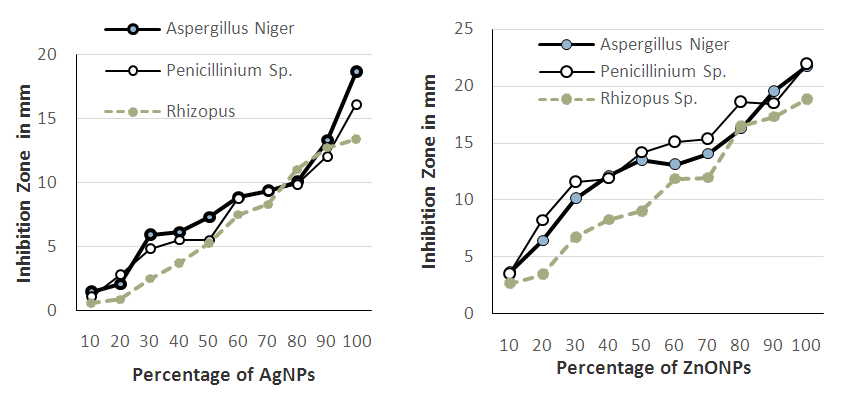 | Figure 5. Antimicrobial Activity of Both Ag and ZnONPs |
3.4. Storage Duration (Shelf Life) of Injera
- The storage duration of Injera without visible mold growth was determined by taking the time from day of baking to the moment in which the samples of a batch show any visible signs of molding. The samples were stored at average temperature of 25.6 ± 2°C and average relative humidity of 50.3 ± 5%. The visible mold sign-free storage period of Injera under unpreserved condition is 3-4 days [3]. Similarly, the current study shows that the shelf life of Injera packed with conventional package was 3 days. The plastic packages coated with Ag and ZnONPs had a better visible mold sign-free storage period (>18 days) at above 70% concentrations. The present results showed that treatments with Ag and ZnO nano particles have a better effect on elongation of Injera shelf life. When comparing Ag and ZnONPs, 10% of ZnONPs has recorded similar result to the control sample at 4th day. However, at same concentration, the shelf life of Injera packed with plastic package coated with AgNPs at 10% was upto 5 days.Also, the result (23 days) from AgNPs at 90% was greater than the result of ZnONPs at 90% (19 days). However, in other treatments (30, 50, 70%), ZnONPs has resulted in higher shelf life than AgNPs. As shown in Fig. 6, the shelf life of Injera samples packaged in plastics with ZnONPs were 8, 15, and 21 days at 30, 50, and 70% concentrations whereas those of AgNPs at same concentrations were 5, 9 and 19th days, respectively.
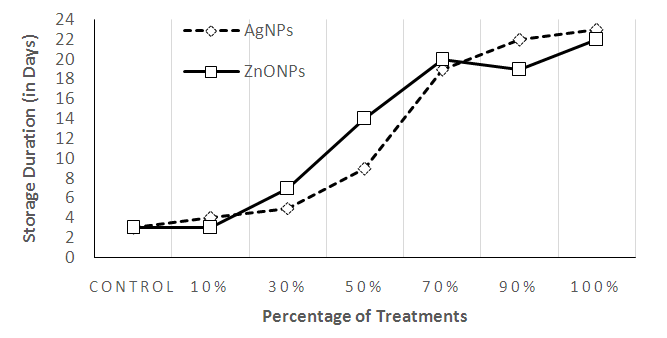 | Figure 6. The Shelf Life of Injera Stored in plastics bag treated with Ag and ZnONPs |
3.5. Moisture Content and pH of the Stored Injera
3.5.1. Moisture Content
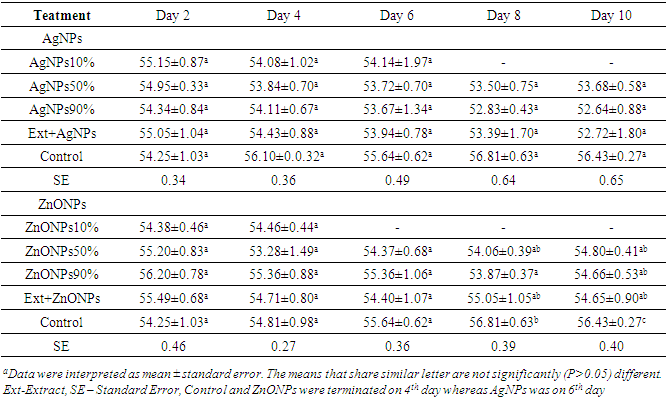
- Results of this investigation showed that there was a significant difference (p<0.05) on the moisture content of Injera samples with different treatments.As showed in Table 1, the moisture content of Injera samples were ranging from 52.6 - 56.7%. The moisture content of Injera samples were decreased. However, after maximum shelf life reached, it starts to be increased that may be due to deterioration caused by overgrowth of fungus.
|
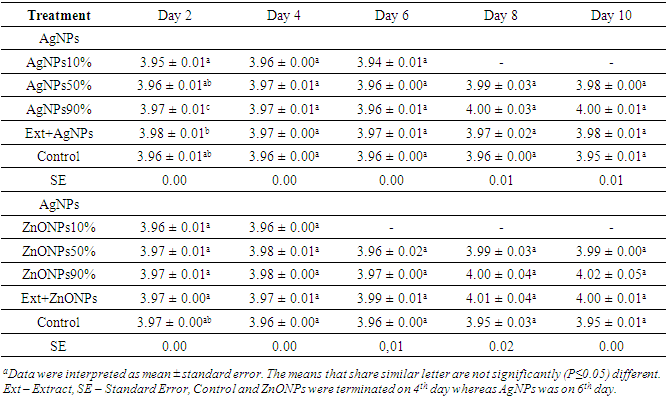
3.5.2. pH of Injera
- As it can be seen from Table 2 that pH of Injera was not significantly (p>0.05) different from the control sample. However, as storage days increases the pH of Injera treated with AgNPs and ZnONPs at different percentages increased, whereas the result from the control (untreated plastic zipper bag) decreased numerically (acidity increased). This increment may be raised from reaction between metal nano particles and other compounds in the Injera samples.
|
3.6. Total Mold and Yeast Count
- In current study, it was also observed that the control sample (uncoated plastic package) had recorded high number of colony forming unit per gram of Injera whereas sample treated with AgNPs or ZnONPs had a low colony-forming unit at each storage day based on their concentration. Even though Injera has only 3-4 days shelf life when packed in conventional packages, it was possible to increase its shelf life to above 20 days using packaging plastic bag treated with Ag and ZnONPs (see Fig 7).
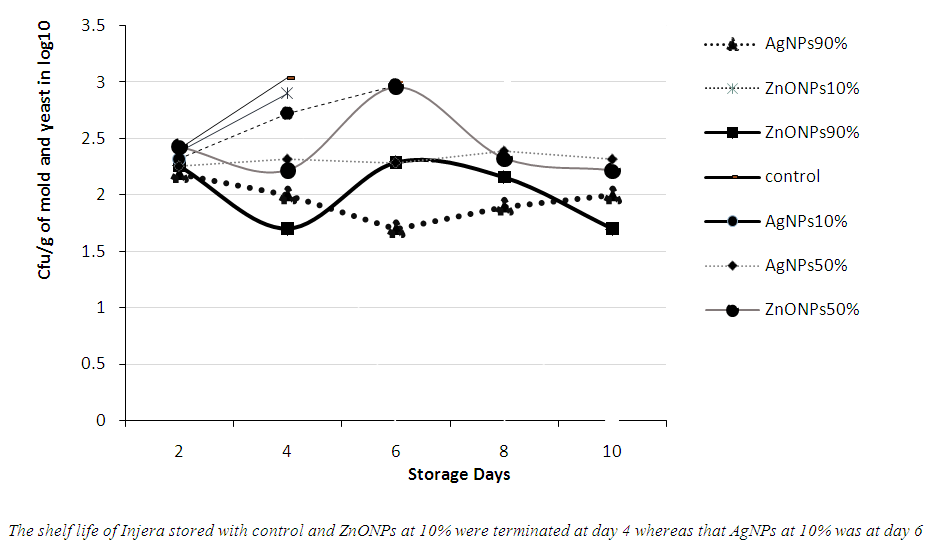 | Figure 7. Colony Count of Mold and Yeast in Stored Injera using plastic coated with Ag and ZnO nano particles |
3.7. Migration of Ag and ZnONPs
- The migration level of the control and AgNPs (10%) were 0 and 0.02 mg/Kg, respectively (Fig 8) whereas other treatments’ results were higher i.e. 0.45, and 0.59 mg/Kg for 50% AgNPs and 90% AgNPs, respectively. The result of the control samples at different days shows similarity showing absence of Ag in tef Injera. At day 2 of storage, 10% AgNPs (0.02 mg/Kg) records lower amount of migration followed by Ext +AgNPs (0.22 mg/kg). There was significant (P ≤ 0.05) difference between each treatments and the control sample except 10% AgNPs. The control sample was significantly different from all other treatments at day 6 and 8 storage days. The results of 10% AgNPs and Ext + AgNPs were similar at both days. But, the results of 50% AgNPs and 90% AgNPs were highly significantly different from other treatments. At day 10, all treatments were statistically different from the control sample. For ZnONPs (Fig 8), there was less migration at day 2. Each treatment was significantly (P≤0.05) different from each other at same storage day. The control sample had about 23.6 Zn (mg/Kg) content whereas treatments at 10, 50 and 90 % had 135.4, 285.6, 348.3 mg/Kg, respectively. Similarly, Ext + ZnONPs has recorded higher migration (511.3 mg/kg) as compared to other treatments. At day 4, the result from the control sample was similar with results at day 2. However, all the other treatments had recorded a higher migration results with their respective order of increased concentration.
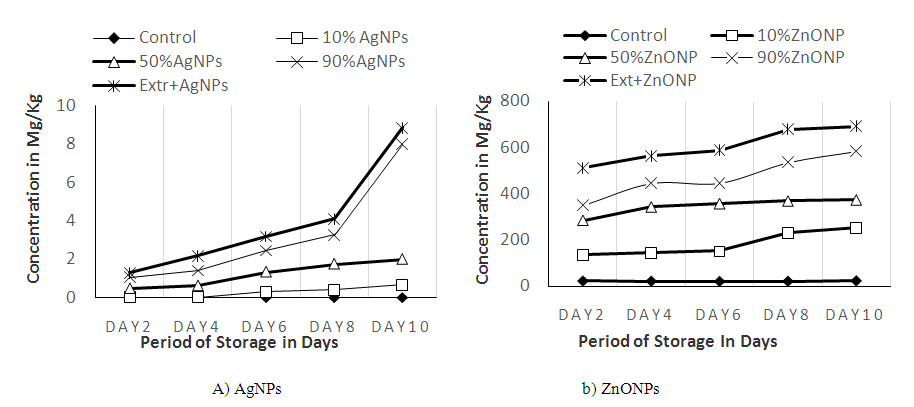 | Figure 8. Migration of Ag+ and Zn2+ to Injera sample stored in plastics coated with Ag and ZnONPs |
4. Conclusions
- Injera is Ethiopian daily stable foods, which is very perishable due to its high moisture content and its acidic nature that favors fast mould and yeast growth. Traditional preservation methods are not convenient for Injera storage. Nano technology is becoming a promising option in elongation of shelf life of food, which can also perform for Injera. In this study, biosynthesized Ag and ZnONPs had better antimicrobial activity against all tested fungus. Aspergillus sp. is more inhibited than Penicillium sp. and Rhizopus sp., respectively. Both NPs increased shelf life of Injera to more than 10 days at concentrations above 50% when packed aseptically. However, the amount of migration of Ag and ZnONPs was relatively high that may be due to direct contact between Injera and dip coated plastic package. The difficulty in using of this dip coated plastics for Injera storage is migration wich is accerated with Injera moisture and transferred to Injera easily. To reduce this migration, it would be advisable to find improved way of using biosynthesized nano particles in packing of Injera which may be inclusion of these nano particles as ingredients of packaging materials during processing.
Conflicts of Interest
- The authors have declared that there is no conflicts of interest in any case.
AKNOWLEDGEMENTS
- The authors thankfully acknowledged Ethiopian Biotechnology Institute, Addis Ababa University School of Food Science and Nutrition and Ethiopian Institute of Agricultural Research for their funding and equipment assistance that helped us to successfully complete this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML