-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2021; 11(2): 35-42
doi:10.5923/j.food.20211102.01
Received: May 15, 2021; Accepted: May 28, 2021; Published: Jun. 15, 2021

Assessment of Minerals, Vitamins and Functional Properties of Flours from Germinated Yellow Maize (Zea mays L.) Seeds from Daloa (Côte D’Ivoire)
Mankambou Jacques Gnanwa1, Jean Bedel Fagbohoun2, Kouamé Claude Ya1, Sika Hortense Blei1, Lucien Patrice Kouame3
1Laboratoire d’Agrovalorisation de l’UFR Agroforesterie, Université Jean Lorougnon Guédé, Daloa, Côte d’Ivoire
2Laboratoire de Biochimie-Génétique, Université Peleforo Gon Coulibaly, Korhogo Côte d'Ivoire
3Laboratoire de Biocatalyse et de Bioprocédés, Université Nanguy Abrogoua, Abidjan, Côte d’Ivoire
Correspondence to: Jean Bedel Fagbohoun, Laboratoire de Biochimie-Génétique, Université Peleforo Gon Coulibaly, Korhogo Côte d'Ivoire.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

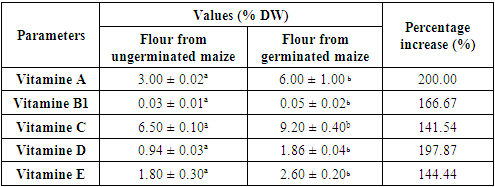
This study was proposed to assess the effect of germination on minerals, vitamins composition and some functional properties of sprouted yellow maize grains with a view to their valorization in the Ivorian diet. The mineral contents (Calcium, Magnesium, Iron, Sodium, Potassium) of sprouted yellow maize samples are statistically different from those of ungerminated maize. Then, it was found that germination resulted in a significant increase in the contents of vitamins (A, B1, C, D and E). The germination of corn kernels led to a significant increase (p <0.05) in the contents of vitamin A (3.00 ± 0.02 to 6.00 ± 1.00 % DW), vitamin B1 (0.03 ± 0.01 to 0.05 ± 0.02 % DW), vitamin C (6.50 ± 0.10 to 9.20 ± 0.40 % DW), vitamin D (0.94 ± 0.03 to 1.86 ± 0.04 % DW) and vitamin E (1.80 ± 0.30 to 2.60 ± 0.20 % DW) from raw and germinated yellow maize respectively. Thus, germination is an effective processing method for increasing vitamins, mineral bio-availability, and for improving significantly functional properties after the yellow maize seeds germinate. Then, germination induced a significant increase in the water and oil absorption capacity (dinor and red) in the flour of the sprouted corn kernels. Therefore, sprouting improves the micronutriments value of corn kernels.
Keywords: Maize, ZeamaysL., Minerals, Vitamins, Functional properties
Cite this paper: Mankambou Jacques Gnanwa, Jean Bedel Fagbohoun, Kouamé Claude Ya, Sika Hortense Blei, Lucien Patrice Kouame, Assessment of Minerals, Vitamins and Functional Properties of Flours from Germinated Yellow Maize (Zea mays L.) Seeds from Daloa (Côte D’Ivoire), International Journal of Food Science and Nutrition Engineering, Vol. 11 No. 2, 2021, pp. 35-42. doi: 10.5923/j.food.20211102.01.
Article Outline
1. Introduction
- Maize (Zea mays L.) is an annual monocotyledonous diploid belonging to the Poaceae family. It is an important cereal that serves as an important food ingredient around the world in the production of varieties of products such as canned corn, breakfast cereals and in the formulation of infant foods (Offia Olua et al., 2020). The genus Zea comprises four species of which Zea mays L. is economically important and is native to Mexico and Central America (Shah et al., 2016). Maize is one of the most widely cultivated plants in the world and the third most important cereal in the world after rice and wheat (Gwirtz & Garcia-Casal, 2014). In Africa, after cassava (Manihot esculenta Crantz) corn is the second most important food crop (Oluwaranti et al., 2015). In Côte d'Ivoire and most of West Africa, maize forms the staple of the diet of rural populations. It is used for human and animal food and is used as raw materials in certain industries (brewing, soap and oil mills) (Hossain et al., 2016). According to Nuss et al. (2011) corn is the most energy-efficient cereal due to its nutritional and economic advantages. In addition, Maize provides more than 20 % of total calories in the human diet in 21 countries and more than 30 % in 12 countries which are home to a total of over 310 million people (Shiferaw et al., 2011; Hossain et al., 2016). In addition, corn is a very interesting and nutritionally edible plant due to its richness in protein as well as certain minerals, carbohydrates and vitamins (Shah et al., 2016). However, the presence of anti-nutritional compounds can affect the digestibility of proteins as well as other nutrients (Andriamasinandraina, 2012; Randrianasolo, 2013). In addition, certain anti-nutritional factors such as α-galactoside, whose fermentation in the colon is largely responsible for gas (Kasprowicz-Potocka et al., 2015; Barkiene et al., 2014; Sathya & Siddhuraju, 2015). This would justify one of the main reasons why people are turning away from the consumption of cereals (corn). However, all of this information on the nutritional potential of corn only concerns the edible portion, that is to say the kernels. Nevertheless, processing techniques such as sprouting improved the quality of cereals due to chemical changes that improve the organoleptic response, the content of free sugars, proteins and vitamins, as well as the bioavailability of minerals and leads to the breakdown of certain endogenous anti-nutritional compounds (Ahmed et al., 2006; Ochanda et al., 2010; Ijarotimi, 2012). Thus, there is constantly an information gap to be filled, especially in terms of the impact of germination technologies on the nutritional value of corn kernels (Vodouhe et al., 2012). This is why the present study was carried out to evaluate the physicochemical composition, some functional properties and some enzymatic activities of sprouted maize grains with a view to their valorization in the Ivorian diet.
2. Materials and Methods
2.1. Raw Material
- Dried grains of yellow maize (Zea mays L.), used as raw material (Figure 1), were purchased at the local market in Daloa (Côte d’Ivoire).
 | Figure 1. Yellow maize (Zea mays L.) |
2.2. Germination Method
- Three hundred grams of the sorted corn sample, disinfected with 1% (v/v) sodium hypochlorite for 10 minutes, is washed thoroughly in tap water and soaked for 24 hours in 500 milliliters of water contained in a 2 liters plastic bucket. They are then spread out on a 100% cotton cloth, and placed in a plastic pot in a room with humidity and temperature of around 85% and 28°C, respectively (Figure 2). Each day, the germinating grains are watered only once. The grains germinated for three days and were prepared for the flours and assay for enzyme activities.
 | Figure 2. Sprouted yellow maize kernels |
2.3. Flour Production
- The corn kernel samples were oven dried for 48 hours at 45°C. They were then ground in a MOULINEX brand mixer to obtain a flour. This flour was stored in pre-dried jars for possible analysis (Figure 3).
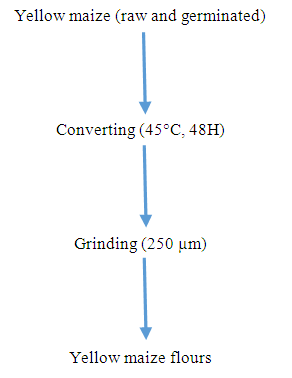 | Figure 3. Flow diagram of the process for producing yellow maize (raw and germinated) flour |
2.4. Mineral Analysis
- The method described by AOAC (1990) was used for mineral analysis. Flours were digested with a mixture of concentrated nitric acid (14.44 mol/L), sulfuric acid (18.01 mol/L) and perchloric acid (11.80 mol/L) and analysed using an atomic absorption spectrophotometer.
2.5. Vitamins Dosage
- Analytical technique used for determination of folate content is HPLC-method (AOAC, 2004).Vitamin C (ascorbic acid) content was determined by the method of Pongracz et al. (1971) and Barros et al. (2007). Vitamin D, vitamin E (α-tocopherol, γ-tocopherol) and carotenoids (provitamin A = β-carotene,) were extracted from 500 mg of yellow maize flour using the following method: 2 mL of distilled water were added to sample. The internal standard (retinyl acetate) was added to the sample in 2 mL of ethanol. The mixture was extracted twice with 8 mL of hexane. After centrifugation (500× g, 10 min at 4°C), 2 mL of distilled water and methanol–dichloromethane (65/35, v/v). A final volume of 150 µL for samples was used for HPLC analysis. The fat-soluble vitamins and carotenoids were separated as previously described (Gleize et al., 2012; Goncalves et al., 2014). All molecules were identified by retention time compared with pure standards.
2.6. Functional Properties Of Samples
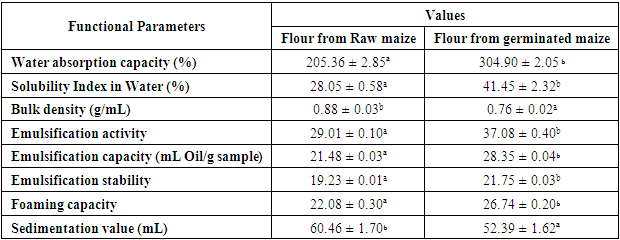
2.6.1. Water Absorption Capacity and Solubility Index in Water
- The water absorption capacity (WAC) of ungerminated and germinated yellow maize flour were determined according to the method of Claver et al. (2010). Distilled water (10ml) was added to 1g of sample, and the mixture was mixed thoroughly using a vortex mixer for 30 min and centrifuge at 4000 rpm for 15 min. The mass of water absorbed was expressed as g/g starch on a dry weight basis. The water absorption capacity and solubility index in water were expressed as percentage increase of the sample weight.
2.6.2. Oil Absorption Capacity
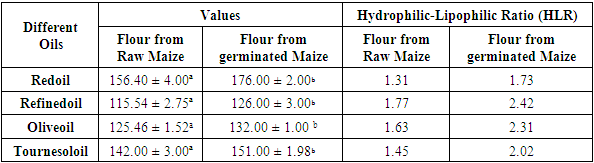
- Oil absorption capacity of the flour samples was determined by the centrifugal method elicited by Eke & Akobundu (1993) with slight modifications. One gram of sample was mixed with 10 ml of oil, the mixture was allowed to stand for 30 min at room temperature, centrifuged at 4000 g for 15 min and the oil that separated was carefully decanted and the tubes were allowed to drain at 45° angle for 10 min and then weighed. Oil absorption was expressed as percentage increase of the sample. And the hydrophilic-lipophilic ratio (HLR) as defined by Njintang et al. (2001).
2.6.3. Foaming capacity and stability
- The procedure of Coffman & Garcia (1977) was used. Three grams of flour sample and 50 mL distilled water were mixed in a Braun blender at room temperature. The suspension was mixed and shaken for 5 minutes at 1600rpm. The content along with the foam was poured into a 100ml graduated measuring cylinder. The total volume was recorded after 30 seconds. Then the content was allowed to stand at room temperature for 30 minutes and the volume of foam only was recorded.
2.6.4. Bulk Density
- Bulk density of the starch was determined according to the method of Musa et al. (2008). Flour (20 g) was weighed into a 50 mL measuring cylinder and the volume occupied was measured and recorded. The cylinder was gently tapped on the bench top 10 times from a height of 5cm. The bulk density was calculated as weight per unit volume of sample.
2.6.5. Dispersibility
- The dispersibility of yellow maize (ungerminated and germinated) flours were measured according to the method of Mora-Escobedo et al. (1991). One gram of the flour was dispersed in distilled water in a 50 mL stoppered measuring cylinder. Then distilled water was added to reach a volume of 30 mL, the mixture was stirred vigorously and allowed to settle for 20 min, the volume of settled particles was subtracted from 30 and multiplied by 100 and reported as percentage dispersibility.
2.6.6. Emulsification Properties
2.6.6.1. Emulsification Capacity
- Emulsification capacity was calculated by the modified method of Naczk et al. (1985). 2.0g of sample was taken and blended with 25 mL distilled water. Corn oil was taken in burette and added to the mixture with continuous blending until the break point was reached (i.e. separation of oil from aqueous phase). The emulsification capacity (EC) was expressed as mL of oil emulsified by 1.0 g of the sample.
2.6.6.2. Emulsification Activity
- Emulsification activity was calculated by the method of Naczk et al. (1985). 3.5 g of flour sample was homogenized in 50 mL water and re-homogenized by adding 50 mL corn oil for 90s. Emulsion was then transferred to two centrifuge tubes equally and centrifuged for 5 min at 1100×g.
2.6.6.3. Emulsion stability
- Emulsion stability was calculated using the above sample after calculating emulsifying activity (Naczk et al., 1985). Sample was heated at 85 ± 2 °C, for 15 minutes and then centrifuged at 1100g for 5 min. Emulsion stability was expressed in percentage as the emulsifying activity remaining after heating using formula for the calculation of emulsion activity (%).
2.7. Statistical Analysis
- All measurements were performed in triplicate. Statistical analyzes of the data were performed using STATISTICA 7 software (Statsoft Inc, Tulsa-USA Headquarters). Comparisons between dependent variables were determined using analysis of variance (one-way ANOVA) and Duncan's test according to the general linear model. The difference between two variables is significant if p ≤ 0.05.
3. Results and Discussion
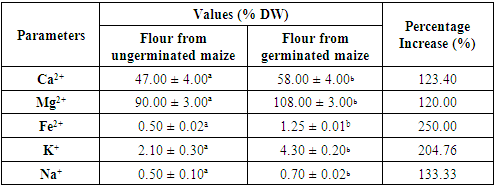
- Mineral CompositionOne of the main reasons for processing foods is to make sure that their nutritional value is maintained over long periods and, where possible, improved. Phytic acid is one of the antinutritional factors common in cereals, which is responsible for binding minerals thus making them not readily bioavailable (Liang et al., 2008). Thus, it should be remembered here that mineral elements are an important group of nutrients necessary for the human body for optimal functioning. The germination of corn kernels significantly increased (p <0.05) the levels of micronutrients such as calcium (47.00 ± 4.00 to 58.00 ± 4.00 % DW), magnesium (90.00 ± 3.00 to 108.00 ± 3.00 % DW), iron (0.50 ± 0.02 to 1.25 ± 0.01 % DW), potassium (2.10 ± 0.30 to 4.30 ± 0.20% DW) and sodium (0.50 ± 0.10 to 0.70 ± 0.02 % DW) presented in table 1. This same observation was observed by Sadawarte et al. (2018) on germinated malt. This finding is believed to be due to the increase in phytase activity during germination which promotes the reduction of phytic acids which bind to minerals and leading to an increase in mineral availability (Luo et al., 2014). In addition, when the seeds are germinated, the minerals chelate or fuse with the proteins, which increases their content (Mostafa, 2013). Moreover, according to Lemmens et al. (2019) the significant increase in the bioaccessibility of minerals attests to the decrease in the content of antinutritional compounds. Thus, one could infer that the germination process is useful in the preparation of nutrient-rich corn. Consequently, the consumption of the present germinated yellow maize seeds would then be recommended for the prevention of certain diseases (Boislève, 2016; Rosique-Esteban et al., 2018; Sadawarte et al., 2018).
|
|
|
|
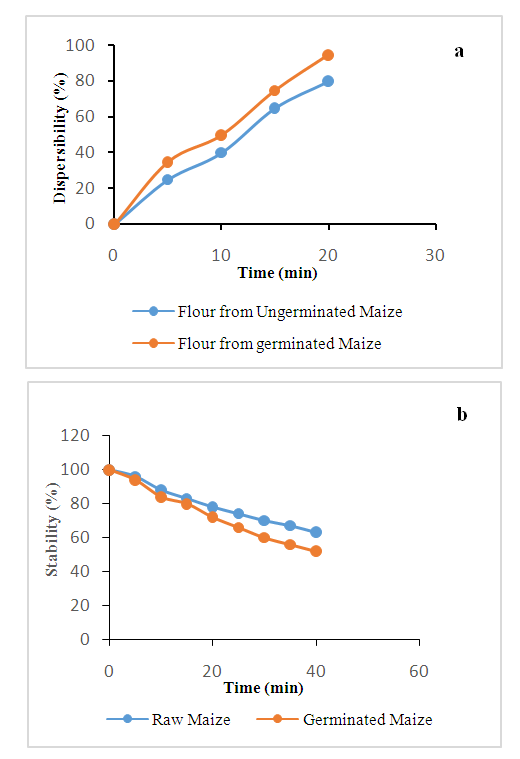 | Figure 4. (a): Dispersibility determination; (b): Foam stability determination of yellow maize flour |
4. Conclusions
- The present study was carried out for the assessment of minerals, vitamins and some functional properties of sprouted maize grains with a view to their valorization in the Ivorian diet. The results of this study revealed that processing techniques such as germination improve levels of certain minerals and vitamins and functional properties of corn kernels. However, flour made from sprouted corn kernels should be supplemented with local fruits and vegetables, which are rich in other vitamins and minerals. With relatively high levels of good functional properties that may be useful in food systems where they can play many functional roles. For example, the water absorption capacity WAC and oil absorption OAC of yellow maize flour make it useful for various products that require water and oil retention for their textural integrity like oil retention capability helps retain flavor and provides good mouth feel. Therefore, the improved functional properties of the germinated yellow maize seed flour could be utilized in food systems where natural modified flour is required rather than chemically or thermally modified white bean seed flour.
Conflict of Interest
- The authors declare that there is no conflict of interest.
Ethical Review
- This study does not involve any human or animal testing.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML