-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2020; 10(2): 43-48
doi:10.5923/j.food.20201002.01
Received: Nov. 20, 2020; Accepted: Dec. 9, 2020; Published: Dec. 15, 2020

Antinutrients, Mineral and Bioavailability Prediction of Calcium and Iron in Eggplant Fruit (Solanum spp.) Varieties Consumed in Côte d’Ivoire
Agnan Marie-Michel Combo1, Patrick Aubin Dakia2, Obouo Hugues Jonathan Gildas Kobi1
1Department of Biochemistry-Microbiology, Laboratory of Agrovalorization, Jean Lorougnon Guédé University, Daloa, Côte d’Ivoire
2Department of Food Science and Technology, Nangui Abrogoua University, Abidjan, Côte d’Ivoire
Correspondence to: Agnan Marie-Michel Combo, Department of Biochemistry-Microbiology, Laboratory of Agrovalorization, Jean Lorougnon Guédé University, Daloa, Côte d’Ivoire.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

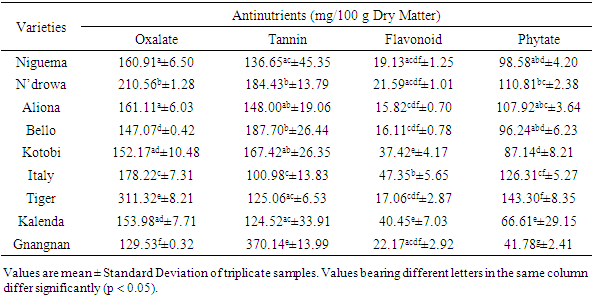
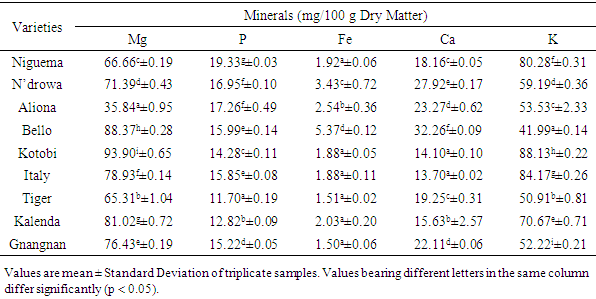
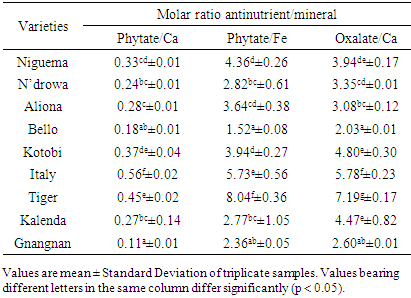
Eggplant is one of the most commonly consumed vegetables in Côte d’Ivoire. For this purpose, the mineral bioavailability and antinutrient constituents of eggplant varieties (Niguema, N’drowa, Aliona, Bello, Kotobi, Italy, Tiger, Kalenda and Gnangnan) were evaluated by standard procedures. The antinutrient composition in dry weight basis was significantly (P < 0.05) varied and ranged: oxalate 311.32-129.53 mg/100 g, tannin 370.14-100.98 mg/100 g, flavonoid 47.35-15.82 mg/100 g, and phytate 143.30-41.78 mg/100 g. Gnangnan had the highest tannin content, Italy had the highest content in flavonoid, while, Tiger presented the highest content in both oxalate and phytate. The results also revealed that eggplant fruits contain some percentage of minerals as follow: Mg (93.90-35.84 mg/100 g), P (19.33-11.70 mg/100 g), Fe (5.37-1.15 mg/100 g), Ca (32.26-13.70 mg/100 g) and K (88.13-41.99 mg/100 g). The calculated molar ratio of phytate/Ca was below the critical value excepted Italy molar ratio, while those of phytate/Fe and oxalate/Ca were above the critical value and this indicate the unavailable of calcium and iron in these studied eggplant varieties. However, this tendency may be reversed if boiling or cooking processes are applied. In view of these results, eggplant antinutritional factors, because of their antioxidant activity being able to have an anti-cancerous property, some authors would recommend to limit their degradation during the technological processes.
Keywords: Eggplant, Mineral, Antinutrient, Bioavailability
Cite this paper: Agnan Marie-Michel Combo, Patrick Aubin Dakia, Obouo Hugues Jonathan Gildas Kobi, Antinutrients, Mineral and Bioavailability Prediction of Calcium and Iron in Eggplant Fruit (Solanum spp.) Varieties Consumed in Côte d’Ivoire, International Journal of Food Science and Nutrition Engineering, Vol. 10 No. 2, 2020, pp. 43-48. doi: 10.5923/j.food.20201002.01.
Article Outline
1. Introduction
- African vegetables, long considered secondary, are nowadays taken into account at national and international levels. This is explained by their nutritional value in the diet of rural areas. Diets rich in vegetables providing micronutrients and health-promoting phytochemicals could alleviate both under-nutrition and obesity [1].Eggplant known as aubergine is a plant belonging to the Solanaceae family and to the genus Solanum [2]. The family Solanaceae is prevalent in tropical, subtropical and Mediterranean areas. Eggplant cultivation is possible in varied climates (temperate, tropical dry or humid). It contains different species and varieties which are distinguished in particular by the color, size and shape of the fruits [3]. It is of great economic importance because several species are cultivated and consumed in the world [4]. World eggplant production is around 50 million tons per year [5].Eggplant contains a significant amount of phytochemicals especially phenolic compounds, such caffeic and chlorogenic acid, and flavonoids, such as nasunin. Eggplant, also contain measurable amounts of oxalates [6]. Some authors reported that eggplant improves digestion, fights constipation, curing cancer, high blood pressure and hepatitis [7,8]. It is effective in the treatment of high blood cholesterol [9].African eggplants include several species including Solanum macrocarpon, S. anguivi and S. aethiopicum. They are in various varietal forms, and bear different vernacular names according to the ethnic groups and the countries. African eggplants are a valuable source of food, in addition to their use for medicinal purposes [10]. In Côte d’Ivoire, eggplants are an essential component that enters in the diet. Eggplant is known for its good palatability and its uses in different dishes. They are important source of vitamins, minerals, dietary fiber and phytochemicals [11]. Moreover, vegetable sources may contain substances harmful for human health, affecting the bioavailability of nutrients. However, to use eggplant as source of nutrients, it is also necessary to demonstrate the presence of antinutritional factors such as tannins, oxalates, phytates etc. Therefore, an attempt was made in the present study to evaluate the mineral and antinutrient compounds of the different eggplant varieties consumed in Côte d’Ivoire.
2. Materials and Methods
- Eggplant fruits, Solanum aethiopicum (Niguema, N’drowa, Kotobi, Aliona and Bello varieties), Solanum melongena (Italy, Tiger and Kalenda varieties) and Solanum anguivi (Gnangnan) used in this study comes from the Laboratory of crop improvement, agroforestry Unit, Jean Lorougnon Guédé University (Daloa, Côte d’Ivoire). The three names Niguema, N’drowa and Gnangnan are from the Akan ethnic group in Côte d’Ivoire. All reagents used in this study were of analytical grade.
2.1. Preparation of Samples
- The eggplant fruits were transported to the laboratory. These fruits were washed with tap water and then with distilled water. The consumable parts were finely cut using a stainless steel knife into rings, oven-dried at 62°C for 24 h, and grounded into fine powder and stored in an airtight container for experimental use. Determinations of minerals and phytochemical constituents were carried out by triplicate. The results were expressed on the basis dry matter (mg/100 g DM).
2.2. Phytochemical Analysis
- Phytochemical constituents were carried out to determine the secondary metabolites present in the samples. The metabolites determined include tannins, oxalates, phytates and flavonoids. Oxalate content was determined using a titration method [12], in which 15 mL of H2SO4 (3 M) were added to 1 g of eggplant powder. After 1 h of homogenization, the solution is filtered through Watman paper. The filtrate is hot-titrated with a solution of KMnO4 (0.05 M) until the pink turn is persistent. Phytate content was determined using the spectrophotometric method of [13], in which the mixture of eggplant powder and 0.65 N HCl (1 g in 20 mL HCl) was stirring for 12 h at room temperature. The mixture obtained is centrifuged at 12000 rpm for 40 min. A volume of 0.5 mL of supernatant was mixed with 3 mL of wade’s reagent. The solution was left to rest for 15 min and the absorbance was measured at 490 nm against blank. A calibration curve was carried out with sodium phytate at 10 mg/mL.Tannin content was determined using the spectrophotometric method described by [14]. 1 mL of the phenolic extract from polyphenol extraction was mixed with 5 mL of vanillin reagent (0.1 mg/mL of vanillin in sulfuric acid 70%). The sample was left to rest for 20 min in the dark and the absorbance was read at 500 nm against blank. Tannin content was estimated using a calibration curve of tannic acid solution (0.1 mg/mL) as standard. Flavonoid content was performed spectrophotometrically according to the method described by [15]. 0.5 mL of the phenolic extract obtained from the extraction of the polyphenols was mixed successively with 0.5 mL of distilled water, 0.5 mL of aluminum chloride (10%, w/v), 5 mL of sodium acetate (1 M) and finally 2 mL of distilled water. The mixture was left to rest for 30 minutes at room temperature and the absorbance was read at 415 nm against a blank. A calibration range was carried from a quercetin solution at 0.1 mg/mL.
2.3. Determination of Mineral Contents
- The mineral content was analyzed on aliquots of the solution of the ash. Magnesium (Mg), phosphorus (P) and potassium (K) were determined using an atomic absorption spectrophotometer (Thermo Scientific iCE 3000 Series) described in previous work [16]. Calcium (Ca) content was carried out by the titrimetric method with EDTA and iron (Fe) content was determined by TPTZ (2,4,6 tripyridyl-5 triazine) method [17].
2.4. Determination of Molar Ratio of Antinutrients to Minerals
- The molar ratio between antinutrient and mineral was obtained according to the method of [18]. Molar ratio = Moles of antinutrient/Moles of mineral = mg antinutrient/MW of antinutrient/ mg mineral/MW of mineral, where MW = atomic weight.
2.5. Statistical Analysis
- The statistical processing of the data consisted of an analysis of variance (ANOVA) with a classification criterion using the Statistica software (Statistica 7.1). Means were compared by the Duncan test at the 5% significance level.
3. Results and Discussion
3.1. Antinutritional Factors of Eggplant Varieties
- The nutritional importance of a given food depends on the nutrients and antinutritional constituents. High concentrations of antinutrients have been discovered to cause great effects on mineral bioavailability in foods [19] by forming complexes with them, as a result reducing their absorption and utilization by the body systems [20]. Phytochemical analysis revealed that the bioactive constituents: oxalate, tannin, flavonoid and phytate were present in all the eggplant varieties (Table 1). However, the major constituent found were oxalate and tannin.
|
3.2. Mineral Contents of Eggplant Varieties
- Minerals are important components of the human diet because they serve as cofactors for many physiological and metabolic processes [28]. The mineral composition of the studied eggplant varieties is presented in Table 2. Analysis of the minerals revealed that the studied eggplants contained high rate of magnesium (Mg), phosphorus (P), iron (Fe), calcium (Ca) and potassium (K). Magnesium and potassium are the minerals with the highest levels, followed by calcium, phosphorus and iron.
|
3.3. Molar Ratio of Antinutrients and Minerals
- To predict the bioavailability of iron and calcium, antinutrient mineral ratios were calculated and compared with the reported critical toxicity values. The calculated molar ratios between antinutrients and minerals of the studied eggplant varieties are showed in Table 3. Results also revealed that there was significantly different in the phytate/Fe, phytate/Ca and oxalate/Ca molar ratio of all the varieties.
|
4. Conclusions
- The present work allows to know the antinutritional, mineral composition and bioavailability of iron and calcium of eggplant fruits consumed in Côte d’Ivoire. The findings obtained shown that eggplants contain high levels of antinutritional factors (oxalate, tannin, flavonoid and phytate) with lower minerals which make iron and calcium unavailable to the human body. However, processing methods such cooking, boiling and other heat treatment would be better to reduce the levels of these antinutrients present in the studied eggplant varieties. Thus, data obtained in this study provided opportunities for further researches concerning the effect of processing on antinutritional components and the bioavailability of minerals in eggplant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML