-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2020; 10(1): 37-41
doi:10.5923/j.food.20201001.03
Received: Aug. 11, 2020; Accepted: Sep. 14, 2020; Published: Dec. 15, 2020

Improving Nutritional Quality of Cowpea (Vigna unguiculata) by Soaking Process
Abdou Diouf1, 2, 3, Fallou Sarr2, 3, Cheikh Ndiaye3, Nicolas Cyrille Ayessou1, Seynabou Momar Fall3
1Centre d’Etude sur la Sécurité Alimentaire et les Molécules Fonctionnelles (CESAM), Université Cheikh Anta DIOP de Dakar, Fann, Sénégal
2Faculté des Sciences et Techniques, Université Cheikh Anta DIOP de Dakar, Fann, Sénégal
3Institut de Technologie Alimentaire (ITA) Route des pères Maristes, Hann, Dakar, Sénégal
Correspondence to: Nicolas Cyrille Ayessou, Centre d’Etude sur la Sécurité Alimentaire et les Molécules Fonctionnelles (CESAM), Université Cheikh Anta DIOP de Dakar, Fann, Sénégal.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Effect of soaking in water and acetic acid solutions to improve nutritional qualities of cowpea was studied. Cowpeas were soaked in tap water, 1 and 2% acetic acid solutions at 4, 8, 16 and 24 hours. Afterwards, phytates and minerals (Fe and Zn) content were quantified. the phytates content of soaked cowpea grain decreased with the acidity of the soaking solution and the duration of treatment. Compared to the raw material, phytates content decrease by 1.48 to 37.59% in samples soaked in tap water at 4 to 24 hours. Likewise, the phytates content decrease by 7.98 to 41.69 in samples soaked in 1 and 2% acetic acid solution at the same soaking time. The best reduction of phytates content (41.69%) is obtained with acetic acid 2% within 24 hours. Regarding minerals, a slight variation with soaking time in their content was obtained. Acetic acid solutions induced a significant removal of zinc but not for iron. The results revealed that for the two treatment, soaking with vinegar for 24h allows a better reduction of phytates with the best bioavailability of iron but low for zinc; soaking in tap water during 24h gives a good bioavailability for iron and a moderate one for zinc.So, we can say that, if intention is to obtain a product rich in iron with less phytates, it is preferable to use 2% acetic acid for 24h which can be adopted in households.
Keywords: Cowpea, Phytates, Soaking, Acetic acid, Minerals
Cite this paper: Abdou Diouf, Fallou Sarr, Cheikh Ndiaye, Nicolas Cyrille Ayessou, Seynabou Momar Fall, Improving Nutritional Quality of Cowpea (Vigna unguiculata) by Soaking Process, International Journal of Food Science and Nutrition Engineering, Vol. 10 No. 1, 2020, pp. 37-41. doi: 10.5923/j.food.20201001.03.
Article Outline
1. Introduction
- The sub-Saharan Africa population’s diet is mainly based on cereals and legumes. Legumes are sources of good quality of protein, carbohydrates, various minerals and vitamins [1]. Having less expensive proteins is an increasing demand across the world and particularly in under-developed countries [2]. Cowpea is one of the main legumes consumed in Senegal which annual production increase from 99924 tons in 2017 to 117784 tons in 2018 [3]. The protein content of cowpea is between 18 and 35 g/100 g [1,4,5]. However, the presence of several antinutritional factors (ANFs) can limit the nutritional values of this legume by reducing the bioavailability of some essential minerals. Phytic acid (phytate; myo-inositol 1,2,3,4,5,6, hexakisphosphate) is one of the ANFs among naturally occurring constituent of plant seeds, roots, tubers, some fruits and vegetables. It acts as a storage form of phosphate [6]. Specifically, phytic acid is known to build complexes with essential dietary minerals such as calcium, zinc, iron and magnesium, making those biologically unavailable for absorption. Phytic acid can also chelate vitamins and potentially contribute to their deficiency and to disease pellagra [7]. In cowpea, phytates content is 559 mg/100g DM [8]. Through previous research, several dipping methods are shown to reduce the phytate content in legume seeds [9,10,11]. Among those methods, soaking, sprouting, fermentation, extrusion cooking and steam pre-cooking are demonstrated [9,11,12]. Soaking cowpea in water during 24 hours lost 8.4% of phytates content [12]. A better reduction of 22.4 and 23.7% on two varieties of cowpea after 24 hours of soaking has been obtained [13]. Unfortunately, these techniques can remove or reduce some recommended components which may be required to enhance nutritional quality [14]. Thus, this study aims to determine optimal conditions which either reduces phytates or preserves nutrients in cowpea.
2. Material and Methods
2.1. Sampling of Cowpea Seeds
- An enough quantity of dry cowpea seeds were purchased from a local food store in Dakar.
2.2. Soaking of Cowpea Seeds
- Cowpea samples were processed from the same way for analyzes. They were soaked in order to reduce phytates in seeds. Two types of solutions were used: tap water and acetic acid (vinegar) at 1 and 2% corresponding to 1 and 2° respectively.Acetic acid is a weak dietary acid which is found in commercial vinegar at 6° ordinary used in households. Treatments were carried out by soaking cowpea in water or acetic acid solutions with the ratio of 1:3 w/v (grain/solution), at room temperature (25°C). Four times soaking were applied: 4, 8, 16 and 24 hours. The pH of tap water was 7.0 and those of the 1 and 2% acetic acid solutions were 4.0 and 3.0 respectively. The samples obtained were named NnT (niébé not treated), NE (niébé soaked in water), NAc1 and NAc2 (niébé soaked in 1% and 2% acetic acid respectively).
2.3. Sample Preparation for Analysis
- Beyond soaking essays, cowpea samples were dried at 75°C in an oven during 24 hours. They were finely grounded to a fine powder (particle size of 0.5 mm) using a laboratory mill 3100 (perten instruments) in order to be analysed.
2.4. Analytical Methods
2.4.1. Phytic Acid Quantification
- Phytic acid content of cowpea was determined using Latta and Eskin [15] and Vaintraub et al. [16] methods with some modifications. 1.2 g of each sample was weighed and introduced into 50 ml tubes. 40 ml of 2.4% concentrated HCl was added to each tube, at room temperature. Tubes were vortexed during 2 hours every 10 min for 15s. After 2 hours, the tubes were centrifuged during 30 min at 3000 rpm. The clear supernatant was used for the phytate quantification. 5 mL of Wade reagent (0.03% solution of FeC13·6H2O containing 0.3% sulfosalicylic acid in water) was added in 15 mL of the supernatant; the mixture is vortexed for 5s. The new supernatant was then transferred in a cell to read absorbance at 500 nm. Distilled water was used as a blank. Phytate content were determined in triplicate.
2.4.2. Zinc and Iron Quantification
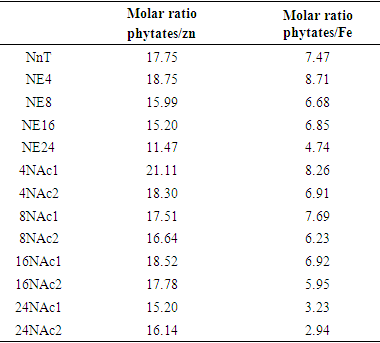
- Minerals content were determined in triplicate. Zinc and Iron contents were determined by atomic absorption spectrophotometry after mineralization at 550°C for 4 hours. Hydrochloric acid was added to the ash obtained and then evaporated to dryness. The residue was dissolved in Nitric acid and this solution was analyzed by Atomic Absorption Spectrophotometer (AAS) using the technique of flame [17]. The molar ratio acid content was calculated using the formula below:
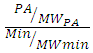 Where: PA = calculated phytate content; MWPA = PA molecular weight (660 Da); Min = mineral content (zinc or iron); MWmin = mineral molecular weight (Zn = 65 Da; Fe = 56 Da).
Where: PA = calculated phytate content; MWPA = PA molecular weight (660 Da); Min = mineral content (zinc or iron); MWmin = mineral molecular weight (Zn = 65 Da; Fe = 56 Da).2.5. Statistical Analysis
- Statistical analysis (XLSAT 6.1.9) was accomplished with the ANOVA coupled with the Fisher test at 95% confidence level. Each sample was analyzed in triplicate. The objective is to compare samples contents through each parameter. Microsoft excel 2013 has been used for graphical illustration.
3. Results and Discussion
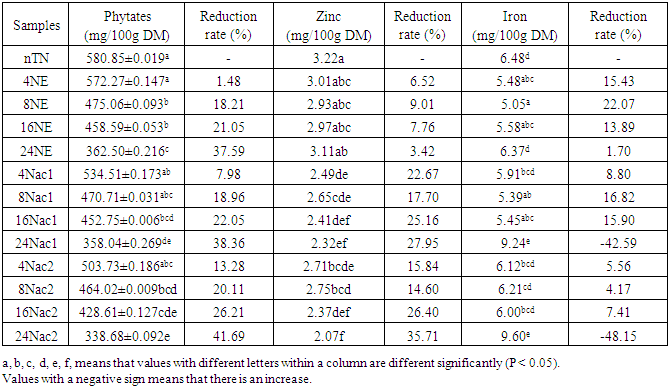
- As shown in Table 1, phytic acid, zinc and iron contents of untreated (not soaked) cowpea are respectively 580.85, 3.22 and 6.48 mg/100g.
|
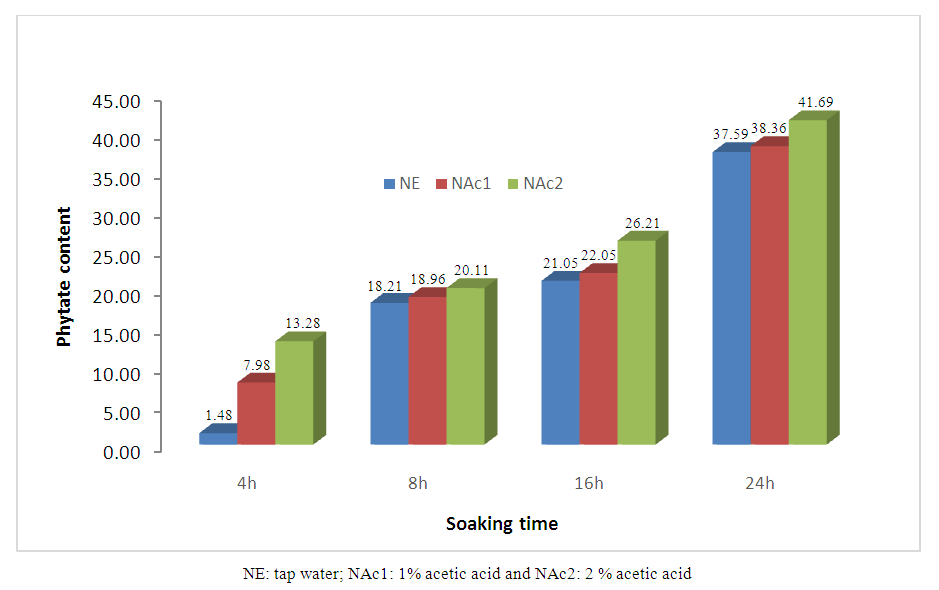 | Figure 1. Rate of phytates reduction during soaking cowpea in variable solutions |
|
4. Conclusions
- Soaking is treatment which is commonly used to prepare cowpea in household. The study reveals that soaking promoted significant phytate reduction in acetic acid solution which can be compare to vinegar. This treatment reduced zinc and iron but did not modified seriously their bioavailability. Then the method can be adopted in households.
ACKNOWLEDGEMENTS
- Funding: USAID Sorghum & Innovation Lab (SMIL AID - OAA - A - 13 -00047) Supported this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML