| [1] | Neeraj, Chittora, A., Bisht, V., and Johar, V. 2017. Marketing and Production of Fruits and Vegetables in India. Int.J.Curr.Microbiol.App.Sci 6, 8, 2896–2907. |
| [2] | Government of India, Ministry of Agriculture. October/2014. Handbook on Horticulture Statistics, New Delhi, INDIA. |
| [3] | Sachdeva, S., Sachdev, T. R., and Sachdeva, R. 2013. Increasing fruit and vegetable consumption: challenges and opportunities. Indian journal of community medicine: official publication of Indian Association of Preventive & Social Medicine 38, 4, 192–197. |
| [4] | Kuiper, M., Kristkova, Z. S., and Grace, D. 2017. The economics of food safety in India – a rapid assessment. KvM 2017-3: Economische kosten FSS India. Wageningen University & Research. |
| [5] | Khare, S., Tonk, A., and Rawat, A. 2018. Foodborne diseases outbreak in India: A review. International Journal of Food Science and Nutrition 3, 3, 9–10. |
| [6] | Prasad, J., Dr. 2017. FOOD-BORNE DISEASES AND FOOD SAFETY IN INDIA. CD Alert, New Delhi, INDIA. |
| [7] | Srivastava, R. K., Dr. 2009. Food-Borne Diseases. CD Alert, New Delhi, INDIA. |
| [8] | Shashi Sareen. 2016. A Scheme and Training Manual on Good Agricultural Practices (GAP) for Fruits and Vegetables. Volume 1 The scheme - standard and implementation infrastructure. FAO, Bangkok. |
| [9] | TNAU Agritech Portal. Good Agricultural Practices (GAP) for fresh Fruits and Vegetables. http://agritech.tnau.ac.in/gap_gmp_glp/gap_fresh%20_%20fruits%20&%20veg.html. |
| [10] | Beuchat, L. R. 1998. Surface decontamination of fruits and vegetables eaten raw: a review WHO/FSF/FOS/98.2. World Health Organization. Food Safety Team & Food and Agriculture Organization of the United Nations. |
| [11] | Anfruns-Estrada, E., Bottaro, M., Pintó, R. M., Guix, S., and Bosch, A. 2019. Effectiveness of Consumers Washing with Sanitizers to Reduce Human Norovirus on Mixed Salad. Foods (Basel, Switzerland) 8, 12. |
| [12] | Mike Kostyo. 2019. 2020 vision: The food trends that will impact the industry in the year ahead. https://www.smartbrief.com/original/2019/12/2020-vision-food-trends-will-impact-industry-year-ahead. Accessed 22-June-2020. |
| [13] | G., E. 2011. Fate of Pesticide Residues on Raw Agricultural Crops after Postharvest Storage and Food Processing to Edible Portions. In Pesticides - Formulations, Effects, Fate, M. Stoytcheva, Ed. InTech. DOI=10.5772/13988. |
| [14] | Huang, Y. and Chen, H. 2015. Inactivation of Escherichia coli O157:H7, Salmonella and human norovirus surrogate on artificially contaminated strawberries and raspberries by water-assisted pulsed light treatment. Food Research International 72, 1–7. |
| [15] | Li, M., Muthaiyan, A., O'Bryan, C. A., Gustafson, J. E., Li, Y., Crandall, P. G., and Ricke, S. C. 2011. Use of natural antimicrobials from a food safety perspective for control of Staphylococcus aureus. Current pharmaceutical biotechnology 12, 8, 1240–1254. |
| [16] | ZHAO, P., ZHAO, T., Doyle, M. P., RUBINO, J. R., and MENG, J. 1998. Development of a Model for Evaluation of Microbial Cross-Contamination in the Kitchen. Journal of food protection 61, 8, 960–963. |
| [17] | Yang, Z., Cao, S., Cai, Y., and Zheng, Y. 2011. Combination of salicylic acid and ultrasound to control postharvest blue mold caused by Penicillium expansum in peach fruit. Innovative Food Science & Emerging Technologies 12, 3, 310–314. |
| [18] | Seymour, I. J. and Appleton, H. 2001. Foodborne viruses and fresh produce. Journal of Applied Microbiology 91, 759–773. |
| [19] | Nasreddine, L. and Parent-Massin, D. 2002. Food contamination by metals and pesticides in the European Union. Should we worry? Toxicology letters 127, 1-3, 29–41. |
| [20] | Tomer, V. and Sangha, J. K. 2013. Vegetable Processing At Household Level: Effective Tool Against Pesticide Residue Exposure. IOSR Journal Of Environmental Science, Toxicology And Food Technology 6, 2, 43–53. |
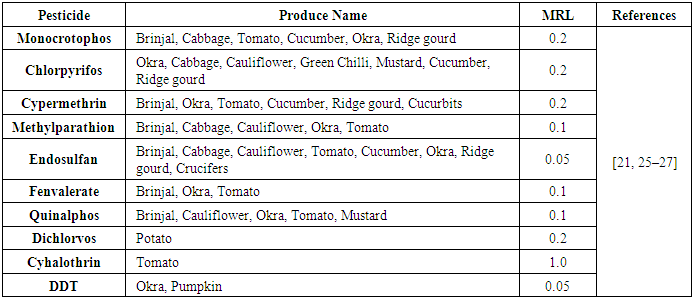
| [21] | Charan, P. D., Ali, S. F., Kachhawa, Y., and Sharma, K. C. 2010. Monitoring of pesticide residues in farmgate vegetables of central Aravalli region of Western India. American-Eurasian Journal of Agricultural & Environmental Sciences 7, 255–258. |
| [22] | Costa, L. G. 2006. Current issues in organophosphate toxicology. Clinica chimica acta; international journal of clinical chemistry 366, 1-2, 1–13. |
| [23] | Chowdhury, M. A. Z., Jahan, I., Karim, N., Alam, M. K., Rahman, M. A., Moniruzzaman, M., Gan, S. H., and Fakhruddin, A. N. M. 2014. Determination of Carbamate and Organophosphorus Pesticides in Vegetable Samples and the Efficiency of Gamma-Radiation in Their Removal. BioMed Research International 2014, 1–9. |
| [24] | Gupta, R. C. 2004. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicology mechanisms and methods 14, 3, 103–143. |
| [25] | Arora, S. 2009. Analysis of insecticides in okra and brinjal from IPM and non-IPM fields. Environmental monitoring and assessment 151, 1-4, 311–315. |
| [26] | Mukherjee, I. 2003. Pesticides residues in vegetables in and around Delhi. Environmental monitoring and assessment 86, 3, 265–271. |
| [27] | RangaRao, G. V., Sahrawat, K. L., Srinivasa, R. C., Binitha, D., Reddy, K. K., and Bharath, B. S. 2009. Insecticide residues in vegetable crops grown in Kothapalli Watershed, Andhra Pradesh, India: A case Study. Indian Journal of Dryland Agricultural Research and Development 24, 21–27. |
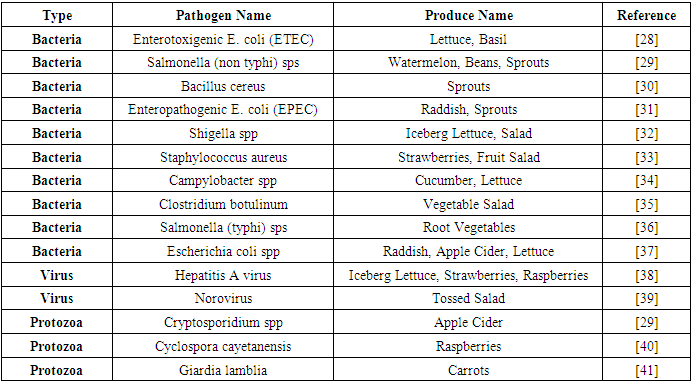
| [28] | Solomon, H. M., Kautter, D. A., Lilly, T., and Rhodehamel, E. J. 1990. Outgrowth of Clostridium botulinum in Shredded Cabbage at Room Temperature Under a Modified Atmosphere. Journal of food protection 53, 10, 831–833. |
| [29] | CDR. 1997. Outbreaks of Escherichia coli O157:H7 Infection and Cryptosporidiosis Associated With Drinking Unpasteurized Apple Cider—Connecticut and New York, October 1996. JAMA 277, 10, 781. |
| [30] | Portnoy, B. L., Goepfert, J. M., and Harmon, S. M. 1976. An outbreak of Bacillus cereus food poisoning resulting from contaminated vegetable sprouts. American journal of epidemiology 103, 6, 589–594. |
| [31] | Lone, A., Anany, H., Hakeem, M., Aguis, L., Avdjian, A.-C., Bouget, M., Atashi, A., Brovko, L., Rochefort, D., and Griffiths, M. W. 2016. Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. International journal of food microbiology 217, 49–58. |
| [32] | Kapperud, G., Rørvik, L. M., Hasseltvedt, V., Høiby, E. A., Iversen, B. G., Staveland, K., Johnsen, G., Leitao, J., Herikstad, H., and Andersson, Y. 1995. Outbreak of Shigella sonnei infection traced to imported iceberg lettuce. Journal of clinical microbiology 33, 3, 609–614. |
| [33] | Fukuyama, S., Watanabe, Y., Kondo, N., Nishinomiya, T., Kawamoto, S., Isshiki, K., and Murata, M. 2009. Efficiency of sodium hypochlorite and calcinated calcium in killing Escherichia coli O157:H7, Salmonella spp., and Staphylococcus aureus attached to freshly shredded cabbage. Bioscience, biotechnology, and biochemistry 73, 1, 9–14. |
| [34] | Kirk, M. D., Pires, S. M., Black, R. E., Caipo, M., Crump, J. A., Devleesschauwer, B., Döpfer, D., Fazil, A., Fischer-Walker, C. L., Hald, T., Hall, A. J., Keddy, K. H., Lake, R. J., Lanata, C. F., Torgerson, P. R., Havelaar, A. H., Angulo, F. J., and Seidlein, L. von. 2015. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med 12, 12, e1001921. |
| [35] | Sockett, P. N., Cowden, J. M., Le Baigue, S., and Ross, D. 1993. Communicable Disease Report. |
| [36] | Kota, S., Govada, V. R., Anantha, R. K., and Verma, M. K. 2017. An Investigation into phytochemical constituents, antioxidant, antibacterial and anti-cataract activity of Alternanthera sessilis , a predominant wild leafy vegetable of South India. Biocatalysis and Agricultural Biotechnology 10, 197–203. |
| [37] | World Health Organization. 1996. Food safety: enterohaemorrhagic Escherichia coli infection. Weekly Epidemiological Record 71, 35, 267–268. |
| [38] | Niu, M. T., Polish, L. B., Robertson, B. H., Khanna, B. K., Woodruff, B. A., Shapiro, C. N., Miller, M. A., Smith, J. D., Gedrose, J. K., and Alter, M. J. 1992. Multistate outbreak of hepatitis A associated with frozen strawberries. The Journal of infectious diseases 166, 3, 518–524. |
| [39] | Lieb, S., Gunn, R. A., Medina, R., Singh, N., May, R. D., Janowski, H. T., and Woodward, W. E. 1985. Norwalk virus gastroenteritis. An outbreak associated with a cafeteria at a college. American journal of epidemiology 121, 2, 259–268. |
| [40] | Herwaldt, B. L. and Ackers, M.-L. 1997. An Outbreak in 1996 of Cyclosporiasis Associated with Imported Raspberries. N Engl J Med 336, 22, 1548–1556. |
| [41] | Mintz, E. D., Hudson-Wragg, M., Mshar, P., Cartter, M. L., and Hadler, J. L. 1993. Foodborne giardiasis in a corporate office setting. The Journal of infectious diseases 167, 1, 250–253. |
| [42] | Thompson, K. 2003. Fruit and Vegetables: Harvesting, Handling and Storage. Postharvest Technology of Fruits and Vegetables. Oxford: Blackwell / Ames, Iowa. |
| [43] | Dakwa, V., Eyles, A., Gracie, A., Tamplin, M., and Ross, T. 2019. Removal of grit from baby leafy salad vegetables by combinations of sanitiser and surfactant. Journal of Food Quality 4. |
| [44] | Michaels, B., Gangar, V., Schattenberg, H., Blevins, M., and Ayers, T. 2003. Effectiveness of cleaning methodologies used for removal of physical, chemical and microbiological residues from produce. Food Serv Technol 3, 1, 9–15. |
| [45] | Borowski, J., Narwojsz, J., Borowska, E. J., and Majewska, K. 2016. The effect of thermal processing on sensory properties, texture attributes, and pectic changes in broccoli. Czech J. Food Sci. 33, No. 3, 254–260. |
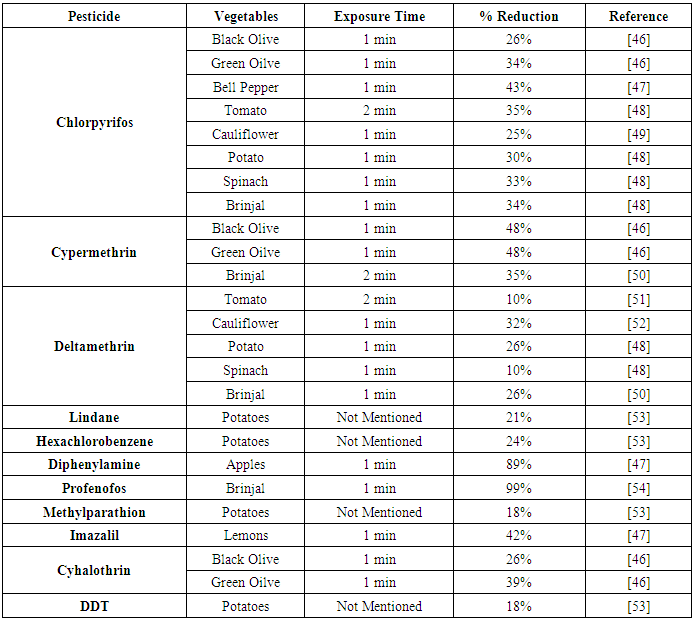
| [46] | Lamia, R. M. and Ali, M. A. 2016. Study the Effect of Household Processing on some Pesticide Residues in Olive Fruits. Middle East Journal of Applied Sciences 6, 3, 588–593. |
| [47] | Al-Taher, F., Chen, Y., Wylie, P., and Cappozzo, J. 2013. Reduction of pesticide residues in tomatoes and other produce. Journal of food protection 76, 3, 510–515. |
| [48] | Randhawa, M. A., Anjum, F. M., Ahmed, A., and Randhawa, M. S. 2007. Field incurred chlorpyrifos and 3,5,6-trichloro-2-pyridinol residues in fresh and processed vegetables. Food Chemistry 103, 3, 1016–1023. |
| [49] | Dhiman, N., Jyot, G., Bakshi, A. K., and Singh, B. 2006. Decontamination of various insecticide in cauliflower and tomato by different processing methods. Journal of Food Science and Technology 43, 1, 92–95. |
| [50] | Kaur, P., Yadav, G. S., Chauhan, R., and Kumari, B. 2011. Persistence of cypermethrin and decamethrin residues in/on brinjal fruits. Bulletin of environmental contamination and toxicology 87, 6, 693–698. |
| [51] | Uysal-Pala, C. and Bilisli, A. 2006. FATE OF ENDOSULFAN AND DELTAMETHRIN RESIDUES DURING TOMATO PASTE PRODUCTION. Journal of Central European Agriculture 7, 2, 343-348. |
| [52] | Panhwar, A. A. and Sheikh, S. A. 2013. Assessment of pesticide residues in cauliflower through gas chromatography-μ ECD and high performance liquid chromatography (HPLC) analysis. International Journal of Agricultural Sciences and Research 3, 1, 7–16. |
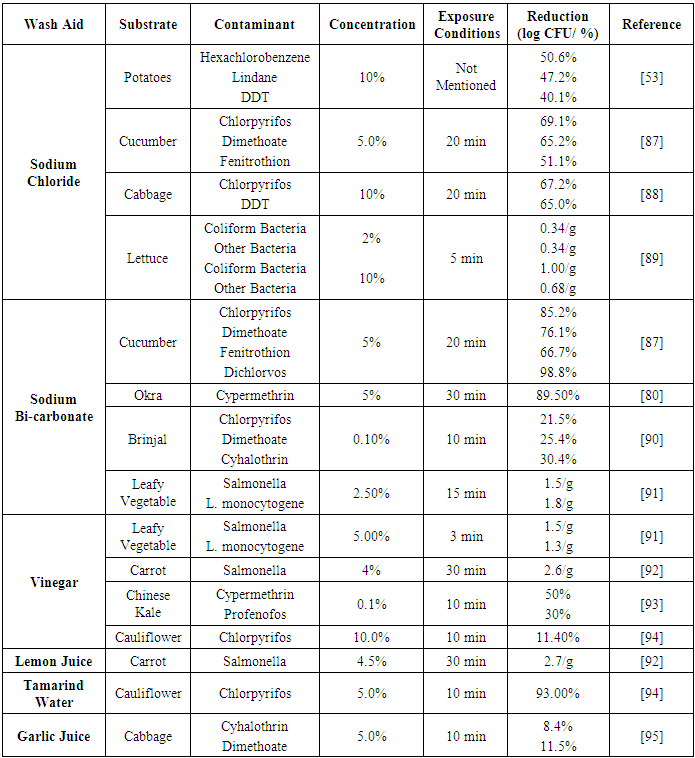
| [53] | Soliman, K. M. 2001. Changes in concentration of pesticide residues in potatoes during washing and home preparation. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 39, 8, 887–891. |
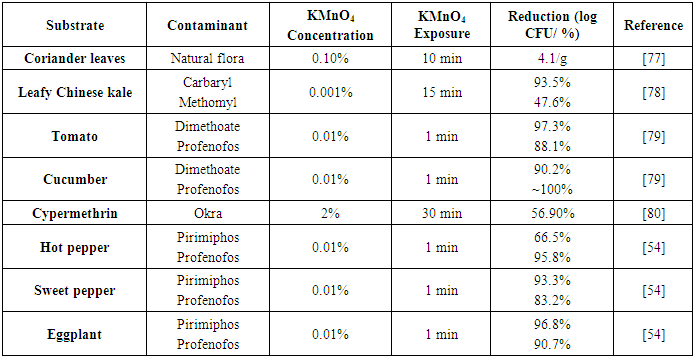
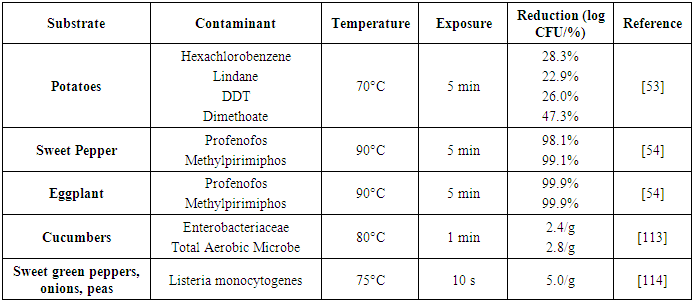
| [54] | Radwan, M. A., Abu-Elamayem, M. M., Shiboob, M. H., and Abdel-Aal, A. 2005. Residual behavior of profenofos on some field-grown vegetables and its removal using various washing solutions and household processing. Food and Chemical Toxicology 43, 4, 553–557. |
| [55] | Croci, L., Medici, D. de, Scalfaro, C., Fiore, A., and Toti, L. 2002. The survival of hepatitis A virus in fresh produce. International journal of food microbiology 73, 29–34. |
| [56] | Baert, L., Debevere, J., and Uyttendaele, M. 2009. The efficacy of preservation methods to inactivate foodborne viruses. International journal of food microbiology 131, 2-3, 83–94. |
| [57] | Dawson, D. J., Paish, A., Staffell, L. M., Seymour, I. J., and Appleton, H. 2005. Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. Journal of Applied Microbiology 98, 203–209. |
| [58] | Sapers, G. M. 2001. Efficacy of Washing and Sanitizing Methods for Disinfection of Fresh Fruit and Vegetable Products. Food Technol. Biotechnol. 39, 4, 305–311. |
| [59] | Satpathy, G., Tyagi, Y. K., and Gupta, R. K. 2012. Removal of Organophosphorus (OP) Pesticide Residues from Vegetables Using Washing Solutions and Boiling. JAS 4, 2. |
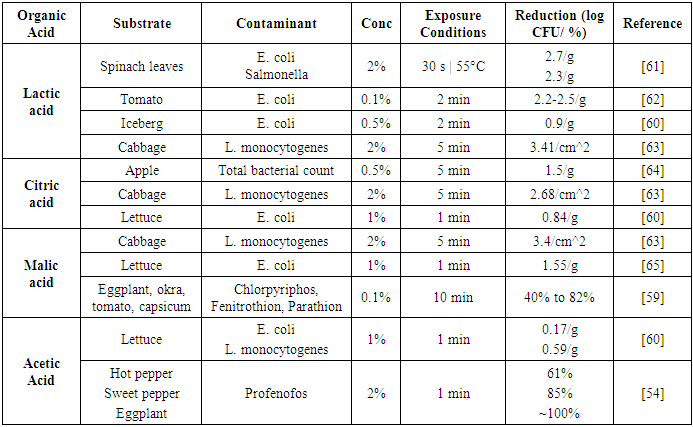
| [60] | Inatsu, Y., Weerakkody, K., Bari, M. L., Hosotani, Y., Nakamura, N., and Kawasaki, S. 2017. The efficacy of combined (NaClO and organic acids) washing treatments in controlling Escherichia coli O157:H7, Listeria monocytogenes and spoilage bacteria on shredded cabbage and bean sprout. LWT - Food Science and Technology 85, 1–8. |
| [61] | Neal, J. A., Marquez-Gonzalez, M., Cabrera-Diaz, E., Lucia, L. M., O'Bryan, C. A., Crandall, P. G., Ricke, S. C., and Castillo, A. 2012. Comparison of multiple chemical sanitizers for reducing Salmonella and Escherichia coli O157:H7 on spinach (Spinacia oleracea) leaves. Food Research International 45, 2, 1123–1128. |
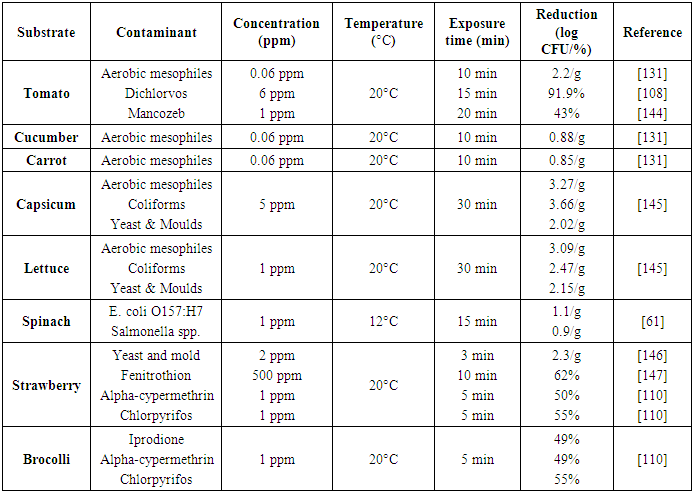
| [62] | Velázquez, L. d. C., Barbini, N. B., Escudero, M. E., Estrada, C. L., and Guzmán, A. M. S. de. 2009. Evaluation of chlorine, benzalkonium chloride and lactic acid as sanitizers for reducing Escherichia coli O157:H7 and Yersinia enterocolitica on fresh vegetables. Food Control 20, 3, 262–268. |
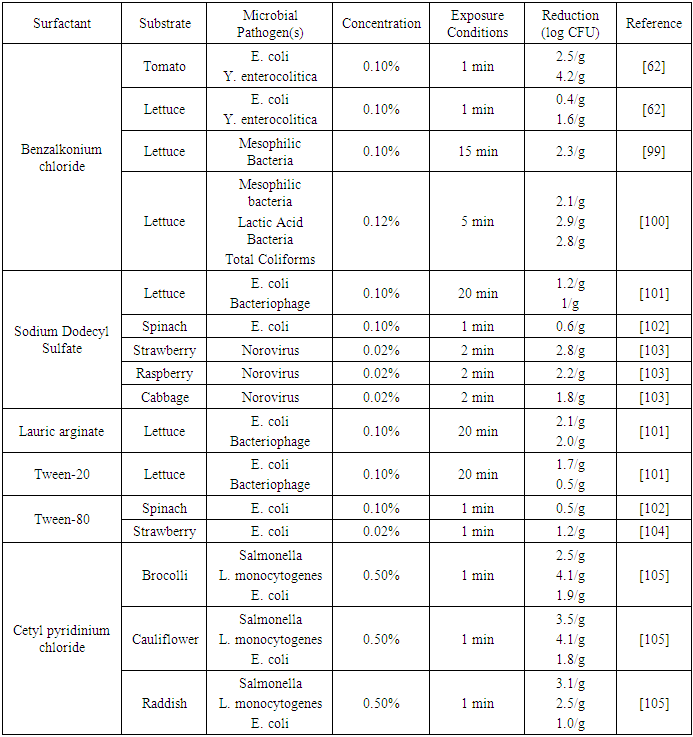
| [63] | Srey, S., Park, S. Y., Jahid, I. K., and Ha, S.-D. 2014. Reduction effect of the selected chemical and physical treatments to reduce L. monocytogenes biofilms formed on lettuce and cabbage. Food Research International 62, 484–491. |
| [64] | Chen, C., Hu, W., He, Y., Jiang, A., and Zhang, R. 2016. Effect of citric acid combined with UV-C on the quality of fresh-cut apples. Postharvest Biology and Technology 111, 126–131. |
| [65] | Sagong, H.-G., Lee, S.-Y., Chang, P.-S., Heu, S., Ryu, S., Choi, Y.-J., and Kang, D.-H. 2011. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. International journal of food microbiology 145, 1, 287–292. |
| [66] | Park, S.-H., Choi, M.-R., Park, J.-W., Park, K.-H., Chung, M.-S., Ryu, S., and Kang, D.-H. 2011. Use of organic acids to inactivate Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. JOURNAL OF FOOD SCIENCE 76, 6, M293-8. |
| [67] | van Haute, S., Uyttendaele, M., and Sampers, I. 2013. Organic acid based sanitizers and free chlorine to improve the microbial quality and shelf-life of sugar snaps. International journal of food microbiology 167, 2, 161–169. |
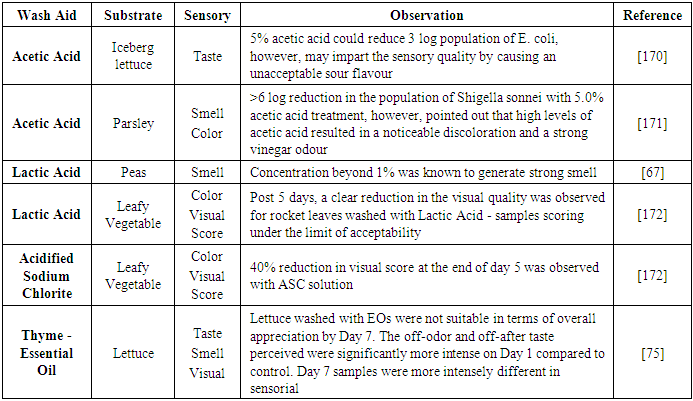
| [68] | Guinoiseau, E., Luciani, A., Rossi, P. G., Quilichini, Y., Ternengo, S., Bradesi, P., and Berti, L. 2010. Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology 29, 7, 873–879. |
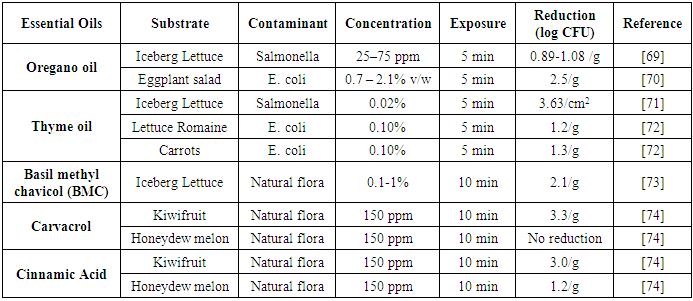
| [69] | Gündüz, G. T., Gönül, Ş. A., and Karapınar, M. 2010. Efficacy of oregano oil in the inactivation of Salmonella typhimurium on lettuce. Food Control 21, 4, 513–517. |
| [70] | Lambert, R. J., Skandamis, P. N., Coote, P. J., and Nychas, G. J. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology 91, 3, 453–462. |
| [71] | Millan-Sango, D., Garroni, E., Farrugia, C., van Impe, J.F.M., and Valdramidis, V. P. 2016. Determination of the efficacy of ultrasound combined with essential oils on the decontamination of Salmonella inoculated lettuce leaves. LWT 73, 80–87. |
| [72] | Singh, N., Singh, R. K., Bhunia, A. K., and Stroshine, R. L. 2002. Efficacy of Chlorine Dioxide, Ozone, and Thyme Essential Oil or a Sequential Washing in Killing Escherichia coli O157:H7 on Lettuce and Baby Carrots. LWT - Food Science and Technology 35, 8, 720–729. |
| [73] | Wan, J., Wilcock, A., and Coventry, M. J. 1998. The effect of essential oils of basil on the growth of Aeromonas hydrophila and Pseudomonas fluorescens. Journal of Applied Microbiology 84, 2, 152–158. |
| [74] | Roller, S. and Seedhar, P. 2002. Carvacrol and cinnamic acid inhibit microbial growth in fresh-cut melon and kiwifruit at 4oC and 8oC. Letters in applied microbiology 35, 390–394. |
| [75] | Gutierrez, J., Bourke, P., Lonchamp, J., and Barry-Ryan, C. 2009. Impact of plant essential oils on microbiological, organoleptic and quality markers of minimally processed vegetables. Innovative Food Science & Emerging Technologies 10, 2, 195–202. |
| [76] | Cleasby, J. L., Baumann, E. R., and Black, C. D. 1964. Effectiveness of Potassium Permanganate for Disinfection. Journal - American Water Works Association 56, 4, 466–474. |
| [77] | Subramanya, S. H., Pai, V., Bairy, I., Nayak, N., Gokhale, S., and Sathian, B. 2018. Potassium permanganate cleansing is an effective sanitary method for the reduction of bacterial bioload on raw Coriandrum sativum. BMC research notes 11, 1, 124. |
| [78] | Klinhom, P., Halee, A., and Methawiwat, S. 2008. The effectiveness of household chemicals in residue removal ofmethomyl and carbaryl pesticides on Chinese kale. Kasesart Journal 42, 136–143. |
| [79] | Shiboob, M. 2012. Residues of Dimethoate and Profenofos in Tomato and Cucumber, and Dissipation During the Removal within Home Processing Method. JKAU: Met., Env. & Arid Land Agric. Sci. 23, 1, 51–63. |
| [80] | Tomer, V., Sangha, J. K., Singh, B., and Takkar, R. 2014. Efficacy of processing treatments on cypermethrin residues in okra (Abelmoschus esculentus). Nutrition & Food Science 44, 6, 545–553. |
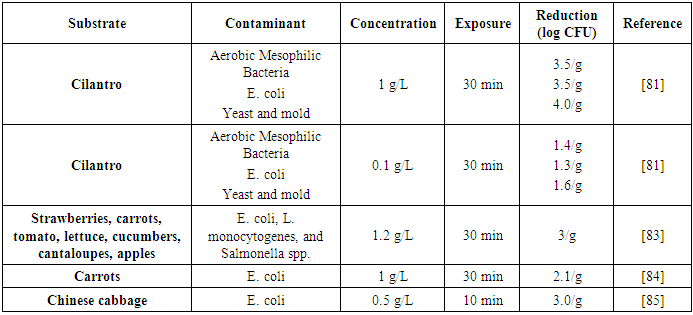
| [81] | Allende, A., McEvoy, J., Tao, Y., and Luo, Y. 2009. Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control 20, 3, 230–234. |
| [82] | BOSILEVAC, J. M., SHACKELFORD, S. D., FAHLE, R., BIELA, T., and KOOHMARAIE, M. 2004. Decreased Dosage of Acidified Sodium Chlorite Reduces Microbial Contamination and Maintains Organoleptic Qualities of Ground Beef Products†. Journal of food protection 67, 10, 2248–2254. |
| [83] | Caldwell, K. N., Adler, B. B., Anderson, G. L., Williams, P. L., and Beuchat, L. R. 2003. Ingestion of Salmonella enterica Serotype Poona by a Free-Living Nematode, Caenorhabditis elegans, and Protection against Inactivation by Produce Sanitizers. AEM 69, 7, 4103–4110. |
| [84] | Gonzalez, R. J., Luo, Y., Ruiz-Cruz, S., and McEvoy, J. L. 2004. Efficacy of sanitizers to inactivate Escherichia coli O157:H7 on fresh-cut carrot shreds under simulated process water conditions. Journal of food protection 67, 11, 2375–2380. |
| [85] | Inatsu, Y., Bari, M. L., Kawasaki, S., Isshiki, K., and Kawamoto, S. 2005. Efficacy of acidified sodium chlorite treatments in reducing Escherichia coli O157:H7 on Chinese cabbage. Journal of food protection 68, 2, 251–255. |
| [86] | Magara, Y., Aizawa, T., Matumoto, N., and Souna, F. 1994. Degradation of pesticides by chlorination during water purification. Water Science and Technology 30, 7, 119–128. |
| [87] | Liang, Y., Wang, W., Shen, Y., Liu, Y., and Liu, X. J. 2012. Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chemistry 133, 3, 636–640. |
| [88] | Zhang, Z.-Y., Liu, X.-J., and Hong, X.-Y. 2007. Effects of home preparation on pesticide residues in cabbage. Food Control 18, 12, 1484–1487. |
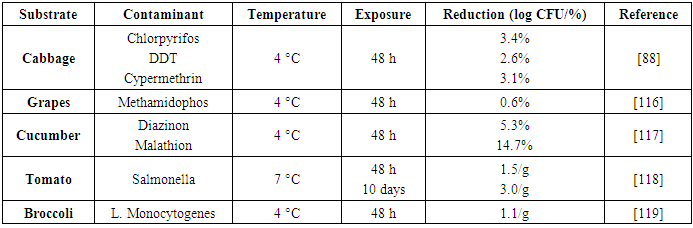
| [89] | Afolabi, O. R. and Oloyede, A. R. 2010. Effectiveness of Chlorinated Water, Sodium Hypochlorite, Sodium Chloride and Sterile Distilled Water in Killing Pathogenic Bacteria on Fresh Produce. Journal of Science & Sustainable Development 3, 1, 29–35. |
| [90] | Ratna Kumari, B., Ranga Rao, G. V., Sahrawat, K. L., and Rajasekhar, P. 2012. Occurrence of Insecticide Residues in Selected Crops and Natural Resources. Bull Environ Contam Toxicol 89, 1, 187–192. |
| [91] | Pezzuto, A., Belluco, S., Losasso, C., Patuzzi, I., Bordin, P., Piovesana, A., Comin, D., Mioni, R., and Ricci, A. 2016. Effectiveness of Washing Procedures in Reducing Salmonella enterica and Listeria monocytogenes on a Raw Leafy Green Vegetable (Eruca vesicaria). Frontiers in microbiology 7, 1663. |
| [92] | Sengun, I. Y. and Karapinar, M. 2004. Effectiveness of lemon juice, vinegar and their mixture in the elimination of Salmonella typhimurium on carrots (Daucus carota L.). International journal of food microbiology 96, 3, 301–305. |
| [93] | Wanwimolruk, S., Kanchanamayoon, O., Phopin, K., and Prachayasittikul, V. 2015. Food safety in Thailand 2: Pesticide residues found in Chinese kale (Brassica oleracea), a commonly consumed vegetable in Asian countries. The Science of the total environment 532, 447–455. |
| [94] | Nowowi, M. F. M., Ishak, M. A. M., Ismail, K., and Zakaria, S. R. 2016. Study on the effectiveness of five cleaning solutions in removing chlorpyrifos residues in cauliflower (Brassica oleracea). Journal of Environmental Chemistry and Ecotoxicology 8, 7, 69–72. |
| [95] | Yu-shan, Z., Xiao-peng, L., Hong-mei, L., Yao-kun, Z., Fan-fei, Z., Qin-jie, Y., and Jian-wen, C. 2013. Study on universal cleaning solution in removing blended pesticide residues in Chinese cabbage. Journal of Environmental Chemistry and Ecotoxicology 5, 8, 202–207. |
| [96] | Ahmad, A., Yeong, S. K., Ismail, R., Ooi, T. L., and Ahmad, S. 2007. Synergistic effect between sodium lauryl sulphate and sodium lauryl ether sulphate with alkyl polyglycoside. JOURNAL OF OIL PALM RESEARCH 19, 332. |
| [97] | Nave, R. Surface Tension of Water. http://hyperphysics.phy-astr.gsu.edu/hbase/surten.html. Accessed 24th June 2020. |
| [98] | Ananthapadmanabhan, K. P. 2019. Amino-Acid Surfactants in Personal Cleansing. Tenside Surfactants Detergents 56, 5, 378–386. |
| [99] | Samadi, N., Abadian, N., Bakhtiari, D., Fazeli, M. R., and Jamalifar, H. 2009. Efficacy of detergents and fresh produce disinfectants against microorganisms associated with mixed raw vegetables. Journal of food protection 72, 7, 1486–1490. |
| [100] | Duarte, A. L. A., do Rosário, D. K. A., Oliveira, S. B. S., Souza, H. L. S. de, Carvalho, R. V. de, Carneiro, J. C. S., Silva, P. I., and Bernardes, P. C. 2018. Ultrasound improves antimicrobial effect of sodium dichloroisocyanurate to reduce Salmonella Typhimurium on purple cabbage. International journal of food microbiology 269, 12–18. |
| [101] | Huang, K. and Nitin, N. 2017. Enhanced removal of Escherichia coli O157:H7 and Listeria innocua from fresh lettuce leaves using surfactants during simulated washing. Food Control 79, 207–217. |
| [102] | Zhang, X., Fan, X., Solaiman, D. K.Y., Ashby, R. D., Liu, Z., Mukhopadhyay, S., and Yan, R. 2016. Inactivation of Escherichia coli O157:H7 in vitro and on the surface of spinach leaves by biobased antimicrobial surfactants. Food Control 60, 158–165. |
| [103] | Predmore, A. and Li, J. 2011. Enhanced removal of a human norovirus surrogate from fresh vegetables and fruits by a combination of surfactants and sanitizers. Applied and environmental microbiology 77, 14, 4829–4838. |
| [104] | KESHUN YU, MELISSA C. NEWMAN, DOUGLAS D. ARCHBOLD, and and THOMAS R. HAMILTON-KEMP. Survival of Escherichia coli O157:H7 on Strawberry Fruit and Reduction of the Pathogen Population by Chemical Agents. |
| [105] | WANG, H., LI, Y., and SLAVIK, M. F. 2001. Efficacy of Cetylpyridinium Chloride in Immersion Treatment for Reducing Populations of Pathogenic Bacteria on Fresh-Cut Vegetables. Journal of food protection 64, 12, 2071–2074. |
| [106] | Xia, J., Xia, Y., and Nnanna, I. A. 1995. Structure-Function Relationship of Acyl Amino Acid Surfactants: Surface Activity and Antimicrobial Properties. J. Agric. Food Chem. 43, 867–871. |
| [107] | Churdchai, C. and Nguyen, D. L. 2010. The Study of Biosurfactant as a Cleaning Agent For Insecticide Residue in Leafy Vegetables*. AU Journal of Technology 14, 2, 75–87. |
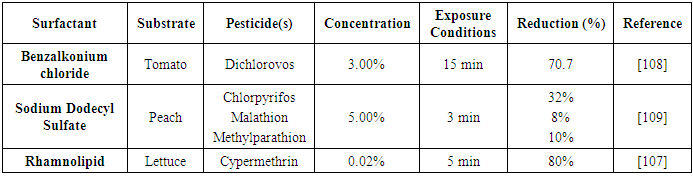
| [108] | HESHMATI, A. and NAZEMI, F. 2018. Dichlorvos (DDVP) residue removal from tomato by washing with tap and ozone water, a commercial detergent solution and ultrasonic cleaner. Food Sci. Technol 38, 3, 441–446. |
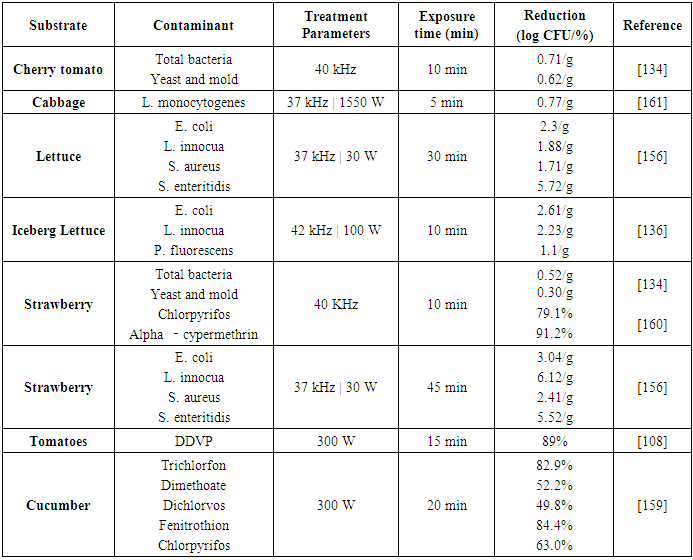
| [109] | Pugliese, P., Moltó, J. C., Damiani, P., Marín, R., Cossignani, L., and Mañes, J. 2004. Gas chromatographic evaluation of pesticide residue contents in nectarines after non-toxic washing treatments. Journal of Chromatography A 1050, 2, 185–191. |
| [110] | Jankowska, M. and Łozowicka, B. 2015. Comparison of the effects of water and thermal processing on pesticide removal in selected fruit and vegetables. J. Elem., 1/2016. |
| [111] | Davis, P. J. and Williams, S. C. 1998. Protein modification by thermal processing. Allergy 53, 46 Suppl, 102–105. |
| [112] | BREIDT, F., HAYES, J. S., and FLEMING, H. P. 2000. Reduction of Microflora of Whole Pickling Cucumbers by Blanching. JOURNAL OF FOOD SCIENCE 65, 8, 1354–1358. |
| [113] | BREIDT, F., HAYES, J. S., and FLEMING, H. P. 2000. Reduction of Microflora of Whole Pickling Cucumbers by Blanching. J Food Science 65, 8, 1354–1358. |
| [114] | Mazzotta, A. S. 2001. Heat resistance of Listeria monocytogenes in vegetables: evaluation of blanching processes. Journal of food protection 64, 3, 385–387. |
| [115] | Wiley, R. C. 1994. Minimally processed refrigerated fruits & vegetables. Chapman & Hall, New York. |
| [116] | Athanasopoulos, P. E., Pappas, C., Kyriakidis, N. V., and Thanos, A. 2005. Degradation of methamidophos on soultanina grapes on the vines and during refrigerated storage. Food Chemistry 91, 2, 235–240. |
| [117] | Mahvi, A. H., Dehghani, M. H., and Vaezi, F. 2005. Ultrasonic Technology Effectiveness in Total Coliforms Disinfection of Water. J. of Applied Sciences 5, 5, 856–858. |
| [118] | Daş, E., Gürakan, G. C., and Bayındırlı, A. 2006. Effect of controlled atmosphere storage, modified atmosphere packaging and gaseous ozone treatment on the survival of Salmonella Enteritidis on cherry tomatoes. Food Microbiology 23, 5, 430–438. |
| [119] | Kozak, G. K., MacDonald, D., Landry, L., and Farber, J. M. 2013. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. Journal of food protection 76, 1, 173–183. |
| [120] | Ramos-Villarroel, A., Y., Aron-Maftei, N., Martín-Belloso, O., and Soliva-Fortuny, R. 2012. Influence of spectral distribution on bacterial inactivation and quality changes of fresh-cut watermelon treated with intense light pulses. Postharvest Biology and Technology 69, 32–39. |
| [121] | Rodgers, S. 2008. Novel applications of live bacteria in food services: Probiotics and protective cultures. Trends in Food Science & Technology 19, 4, 188–197. |
| [122] | Arevalos-Sánchez, M., Regalado, C., Martin, S., E., Domínguez-Domínguez, J., and García-Almendárez, B., E. 2012. Effect of neutral electrolyzed water and nisin on Listeria monocytogenes biofilms, and on listeriolysin O activity. Food Control 24, 1-2, 116–122. |
| [123] | Goodburn, C. and Wallace, C. A. 2013. The microbiological efficacy of decontamination methodologies for fresh produce: A review. Food Control 32, 2, 418–427. |
| [124] | Pino, N. and Peñuela, G. 2011. Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site. International Biodeterioration & Biodegradation 65, 6, 827–831. |
| [125] | Carlton, R. M., Noordman, W. H., Biswas, B., Meester, E. D. de, and Loessner, M. J. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regulatory toxicology and pharmacology : RTP 43, 3, 301–312. |
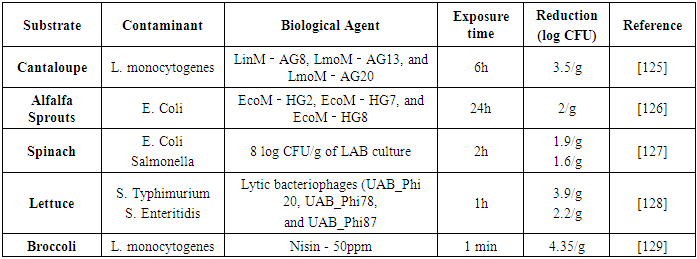
| [126] | Lone, A., Anany, H., Hakeem, M., Aguis, L., Avdjian, A.-C., Bouget, M., Atashi, A., Brovko, L., Rochefort, D., and Griffiths, M. W. 2016. Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. International journal of food microbiology 217, 49–58. |
| [127] | Cálix-Lara, T. F., Rajendran, M., Talcott, S. T., Smith, S. B., Miller, R. K., Castillo, A., Sturino, J. M., and Taylor, T. M. 2014. Inhibition of Escherichia coli O157:H7 and Salmonella enterica on spinach and identification of antimicrobial substances produced by a commercial Lactic Acid Bacteria food safety intervention. Food Microbiology 38, 192–200. |
| [128] | Spricigo, D. A., Bardina, C., Cortés, P., and Llagostera, M. 2013. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. International journal of food microbiology 165, 2, 169–174. |
| [129] | Bari, M. L., Ukuku, D. O., Kawasaki, T., Inatsu, Y., Isshiki, K., and Kawamoto, S. 2005. Combined efficacy of nisin and pediocin with sodium lactate, citric acid, phytic acid, and potassium sorbate and EDTA in reducing the Listeria monocytogenes population of inoculated fresh-cut produce. Journal of food protection 68, 7, 1381–1387. |
| [130] | Rahman, S. M.E., Khan, I., and Oh, D.-H. 2016. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Comprehensive Reviews in Food Science and Food Safety 15, 3, 471–490. |
| [131] | Bhilwadikar, T., Pounraj, S., Manivannan, S., Rastogi, N. K., and Negi, P. S. 2019. Decontamination of Microorganisms and Pesticides from Fresh Fruits and Vegetables: A Comprehensive Review from Common Household Processes to Modern Techniques. Comprehensive Reviews in Food Science and Food Safety 18, 4, 1003–1038. |
| [132] | Graça, A., Abadias, M., Salazar, M., and Nunes, C. 2011. The use of electrolyzed water as a disinfectant for minimally processed apples. Postharvest Biology and Technology 61, 2-3, 172–177. |
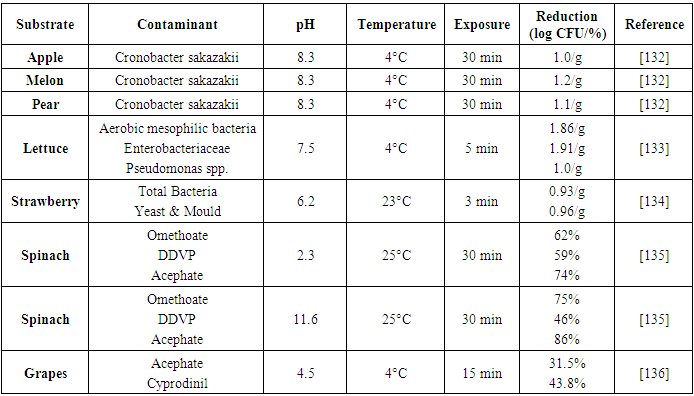
| [133] | Pinto, L., Ippolito, A., and Baruzzi, F. 2015. Control of spoiler Pseudomonas spp. on fresh cut vegetables by neutral electrolyzed water. Food Microbiology 50, 102–108. |
| [134] | Ding, T., Ge, Z., Shi, J., Xu, Y.-T., Jones, C. L., and Liu, D.-H. 2015. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT 60, 2, 1195–1199. |
| [135] | Hao, J., Wuyundalai, Liu, H., Chen, T., Zhou, Y., Su, Y.-C., and Li, L. 2011. Reduction of pesticide residues on fresh vegetables with electrolyzed water treatment. JOURNAL OF FOOD SCIENCE 76, 4, C520-4. |
| [136] | Qi, H., Huang, Q., and Hung, Y.-C. 2018. Effectiveness of electrolyzed oxidizing water treatment in removing pesticide residues and its effect on produce quality. Food Chemistry 239, 561–568. |
| [137] | Suslow, T. V. 2004. Ozone Applications for Postharvest Disinfection of Edible Horticultural Crops. University of California, Agriculture and Natural Resources. |
| [138] | Xu, L. 1999. Use of Ozone to Improve the Safety of Fresh Fruits and Vegetables. Food Technology Magazine 53, 10, 58–63. |
| [139] | Langlais, B., Reckhow, D., A., and Brink, D., R. 1991. Practical application of ozone: Principle and case study. Ozone in Water Treatment. Lewis Publishers, Chelsea, Mich. |
| [140] | Sapers, G. M. 1998. New technologies for safer produce-chemical based treatments and decontamination by washing. Proceedings of Fresh Fruits and Vegetables. Food Safety Challenges, 12–14. |
| [141] | Karaca, H. and Velioglu, Y. S. 2007. Ozone Applications in Fruit and Vegetable Processing. Food Reviews International 23, 1, 91–106. |
| [142] | Dennis, R., Cashion, A., Emanuel, S., and Hubbard, D. 2020. Ozone Gas: Scientific Justification and Practical Guidelines for Improvised Disinfection using Consumer-Grade Ozone Generators and Plastic Storage Boxes. JoSaM 2, 1. |
| [143] | Rice, Rip, G. and Graham, Dee, M. 2001. US FDA regulatory approval of ozone as an antimicrobial agent–what is allowed and what needs to be understood. Ozone News 29, 5, 22–31. |
| [144] | Cengiz, Mehmet, Fatih and Certel, M. 2014. Effects of chlorine, hydrogen peroxide, and ozone on the reduction of mancozeb residues on tomatoes. Turkish Journal of Agriculture and Forestry 38, 3, 371–376. |
| [145] | Alexopoulos, A., Plessas, S., Ceciu, S., Lazar, V., Mantzourani, I., Voidarou, C., Stavropoulou, E., and Bezirtzoglou, E. 2013. Evaluation of ozone efficacy on the reduction of microbial population of fresh cut lettuce (Lactuca sativa) and green bell pepper (Capsicum annuum). Food Control 30, 2, 491–496. |
| [146] | Alexandre, E. M.C., Brandão, T. R.S., and Silva, C. L.M. 2012. Assessment of the impact of hydrogen peroxide solutions on microbial loads and quality factors of red bell peppers, strawberries and watercress. Food Control 27, 2, 362–368. |
| [147] | Ikeura, H., Kobayashi, F., and Tamaki, M. 2011. Removal of residual pesticide, fenitrothion, in vegetables by using ozone microbubbles generated by different methods. Journal of Food Engineering 103, 3, 345–349. |
| [148] | SAMS, A. R. and FERIA, R. 1991. Microbial Effects of Ultrasonication of Broiler Drumstick Skin. J Food Science 56, 1, 247–248. |
| [149] | Rastogi, N. K. 2011. Opportunities and challenges in application of ultrasound in food processing. Critical reviews in food science and nutrition 51, 8, 705–722. |
| [150] | Butz, P. and Tauscher, B. 2002. Emerging technologies: chemical aspects. Food Research International 35, 2-3, 279–284. |
| [151] | Brilhante São José, J. F. and Dantas Vanetti, M. C. 2012. Effect of ultrasound and commercial sanitizers in removing natural contaminants and Salmonella enterica Typhimurium on cherry tomatoes. Food Control 24, 1-2, 95–99. |
| [152] | Mahvi, A. H., Dehghani, M., H., and Vaezi, F. 2005. Ultrasonic Technology Effectiveness in Total Coliforms Disinfection of Water. J. of Applied Sciences 5, 5, 856–858. |
| [153] | Atchley, A. A., Frizzell, L. A., Apfel, R. E., Holland, C. K., Madanshetty, S., and Roy, R. A. 1988. Thresholds for cavitation produced in water by pulsed ultrasound. Ultrasonics 26, 5, 280–285. |
| [154] | Kentish, S. and Ashokkumar, M. 2011. The Physical and Chemical Effects of Ultrasound. In Ultrasound Technologies for Food and Bioprocessing, H. Feng, G. Barbosa-Canovas and J. Weiss, Eds. Food Engineering Series. Springer New York, New York, NY, 1–12. DOI=10.1007/978-1-4419-7472-3_1. |
| [155] | Hulsmans, A., Joris, K., Lambert, N., Rediers, H., Declerck, P., Delaedt, Y., Ollevier, F., and Liers, S. 2010. Evaluation of process parameters of ultrasonic treatment of bacterial suspensions in a pilot scale water disinfection system. Ultrasonics sonochemistry 17, 6, 1004–1009. |
| [156] | Birmpa, A., Sfika, V., and Vantarakis, A. 2013. Ultraviolet light and ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. International journal of food microbiology 167, 1, 96–102. |
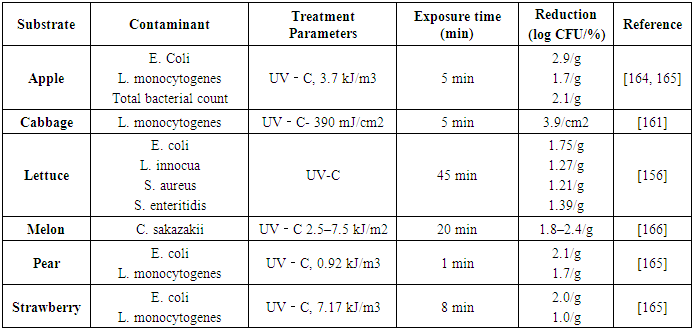
| [157] | López-Malo, A., Palou, E., Jiménez-Fernández, M., Alzamora, S. M., and Guerrero, S. 2005. Multifactorial fungal inactivation combining thermosonication and antimicrobials. Journal of Food Engineering 67, 1-2, 87–93. |
| [158] | Guerrero, S., Tognon, M., and Alzamora, S. M. 2005. Response of Saccharomyces cerevisiae to the combined action of ultrasound and low weight chitosan. Food Control 16, 2, 131–139. |
| [159] | Liang, Y., Wang, W., Shen, Y., Liu, Y., and Liu, X. J. 2012. Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chemistry 133, 3, 636–640. |
| [160] | Lozowicka, B., Jankowska, M., Hrynko, I., and Kaczynski, P. 2016. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environmental monitoring and assessment 188, 1, 51. |
| [161] | Srey, S., Park, S. Y., Jahid, I. K., and Ha, S.-D. 2014. Reduction effect of the selected chemical and physical treatments to reduce L. monocytogenes biofilms formed on lettuce and cabbage. Food Research International 62, 484–491. |
| [162] | Turtoi, M. 2013. Ultraviolet light treatment of fresh fruits and vegetables surface: A review. Journal of Agroalimentary Processes and technologies 19, 3, 325–337. |
| [163] | Food and Drug Administration. 2018. Code of Federal Regulations. Title 21 CFR section 179.39. |
| [164] | Liu, C., Li, X., and Chen, H. 2015. Application of water-assisted ultraviolet light processing on the inactivation of murine norovirus on blueberries. International journal of food microbiology 214, 18–23. |
| [165] | Adhikari, A., Syamaladevi, R. M., Killinger, K., and Sablani, S. S. 2015. Ultraviolet-C light inactivation of Escherichia coli O157:H7 and Listeria monocytogenes on organic fruit surfaces. International journal of food microbiology 210, 136–142. |
| [166] | Santo, D., Graça, A., Nunes, C., and Quintas, C. 2016. Survival and growth of Cronobacter sakazakii on fresh-cut fruit and the effect of UV-C illumination and electrolyzed water in the reduction of its population. International journal of food microbiology 231, 10–15. |
| [167] | Bagchi, D. and Swaroop, A. 2016. Food Toxicology. CRC Press LLC; CRC Press, [Place of publication not identified]. |
| [168] | Olaimat, A. N. and Holley, R. A. 2012. Factors influencing the microbial safety of fresh produce: a review. Food Microbiology 32, 1, 1–19. |
| [169] | Upadhyay, R. and Nishant, N. 2016. Presence of pesticide residue in vegetable crops: A review. AG 37, 3. |
| [170] | Small, D. A., Chang, W., Toghrol, F., and Bentley, W. E. 2007. Comparative global transcription analysis of sodium hypochlorite, peracetic acid, and hydrogen peroxide on Pseudomonas aeruginosa. Applied microbiology and biotechnology 76, 5, 1093–1105. |
| [171] | Wu, F. M., Doyle, M. P., Beuchat, L. R., Wells, J. G., Mintz, E. D., and Swaminathan, B. 2000. Fate of Shigella sonnei on parsley and methods of disinfection. Journal of food protection 63, 5, 568–572. |
| [172] | Martínez-Sánchez, A., Allende, A., Bennett, R. N., Ferreres, F., and Gil, M. I. 2006. Microbial, nutritional and sensory quality of rocket leaves as affected by different sanitizers. Postharvest Biology and Technology 42, 1, 86–97. |
| [173] | Banach, J. L., Sampers, I., van Haute, S., and van der Fels-Klerx, H. J. I. 2015. Effect of Disinfectants on Preventing the Cross-Contamination of Pathogens in Fresh Produce Washing Water. International journal of environmental research and public health 12, 8, 8658–8677. |
| [174] | Xiao, D., Ye, R., Davidson, P. M., Hayes, D. G., Golden, D. A., and Zhong, Q. 2011. Sucrose monolaurate improves the efficacy of sodium hypochlorite against Escherichia coli O157:H7 on spinach. International journal of food microbiology 145, 1, 64–68. |
| [175] | Leistner, L. 2000. Basic aspects of food preservation by hurdle technology. International journal of food microbiology 55, 1-3, 181–186. |
| [176] | Rahman, M. S. 2015. Hurdle Technology in Food Preservation. In Minimally Processed Foods, M. W. Siddiqui and M. S. Rahman, Eds. Food Engineering Series. Springer International Publishing, Cham, 17–33. DOI=10.1007/978-3-319-10677-9_2. |
| [177] | Yuk, H.-G., Yoo, M.-Y., Yoon, J.-W., Moon, K.-D., Marshall, D. L., and Oh, D.-H. 2006. Effect of Combined Ozone and Organic Acid Treatment for Control of Escherichia coli O157:H7 and Listeria monocytogenes on Lettuce. J Food Science 71, 3, M83-M87. |
| [178] | Huang, Y. and Chen, H. 2015. Inactivation of Escherichia coli O157:H7, Salmonella and human norovirus surrogate on artificially contaminated strawberries and raspberries by water-assisted pulsed light treatment. Food Research International 72, 1–7. |
| [179] | Millan-Sango, D., Garroni, E., Farrugia, C., van Impe, J.F.M., and Valdramidis, V. P. 2016. Determination of the efficacy of ultrasound combined with essential oils on the decontamination of Salmonella inoculated lettuce leaves. LWT 73, 80–87. |
| [180] | Forghani, F. and Oh, D.-H. 2013. Hurdle enhancement of slightly acidic electrolyzed water antimicrobial efficacy on Chinese cabbage, lettuce, sesame leaf and spinach using ultrasonication and water wash. Food Microbiology 36, 1, 40–45. |
| [181] | Sagong, H.-G., Lee, S.-Y., Chang, P.-S., Heu, S., Ryu, S., Choi, Y.-J., and Kang, D.-H. 2011. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. International journal of food microbiology 145, 1, 287–292. |
| [182] | Duarte, A. L. A., do Rosário, D. K. A., Oliveira, S. B. S., Souza, H. L. S. de, Carvalho, R. V. de, Carneiro, J. C. S., Silva, P. I., and Bernardes, P. C. 2018. Ultrasound improves antimicrobial effect of sodium dichloroisocyanurate to reduce Salmonella Typhimurium on purple cabbage. International journal of food microbiology 269, 12–18. |
| [183] | Chen, C., Hu, W., He, Y., Jiang, A., and Zhang, R. 2016. Effect of citric acid combined with UV-C on the quality of fresh-cut apples. Postharvest Biology and Technology 111, 126–131. |


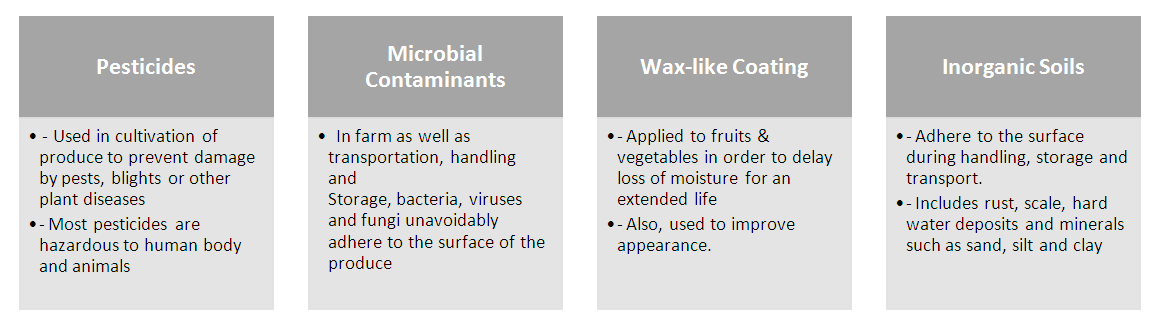

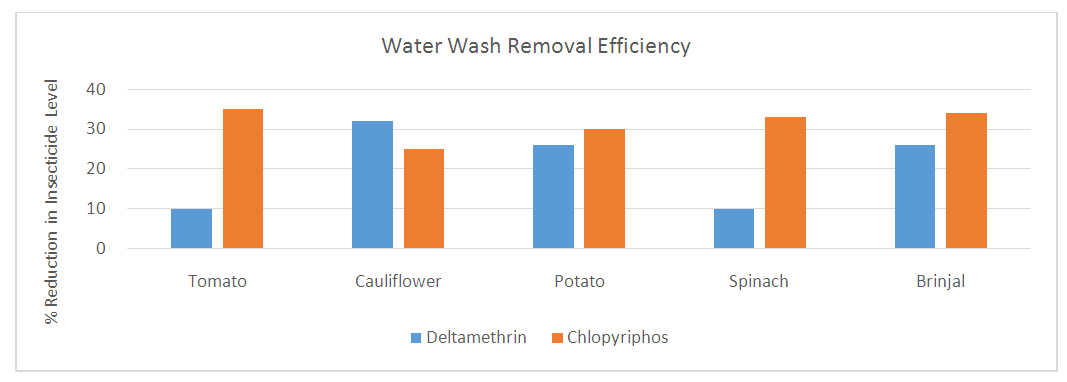
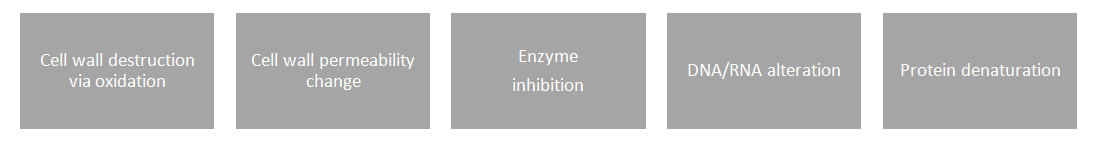
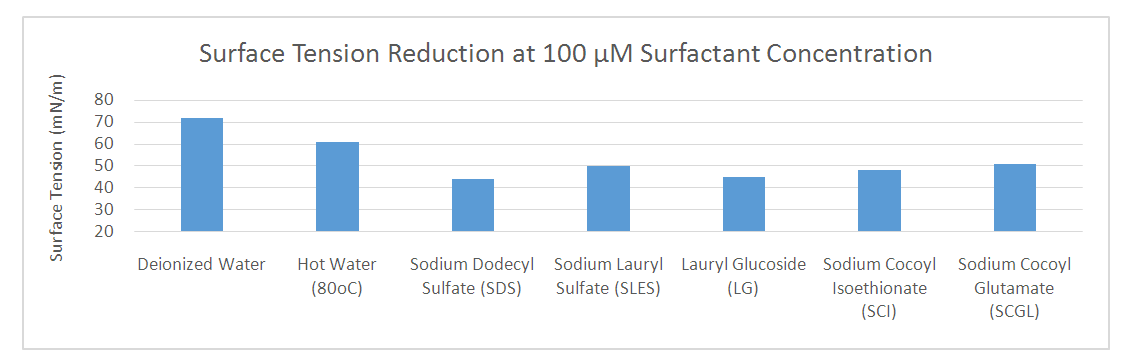
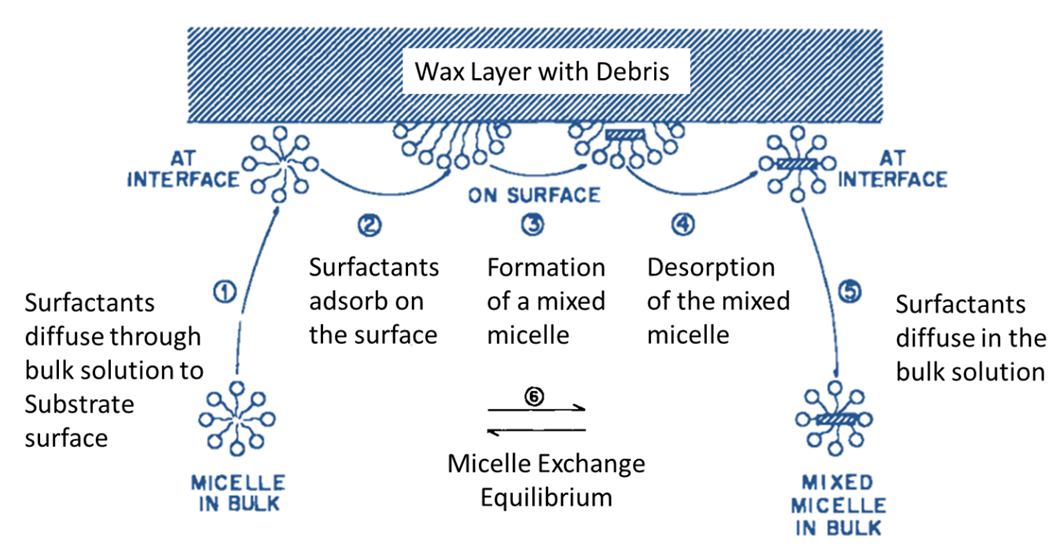
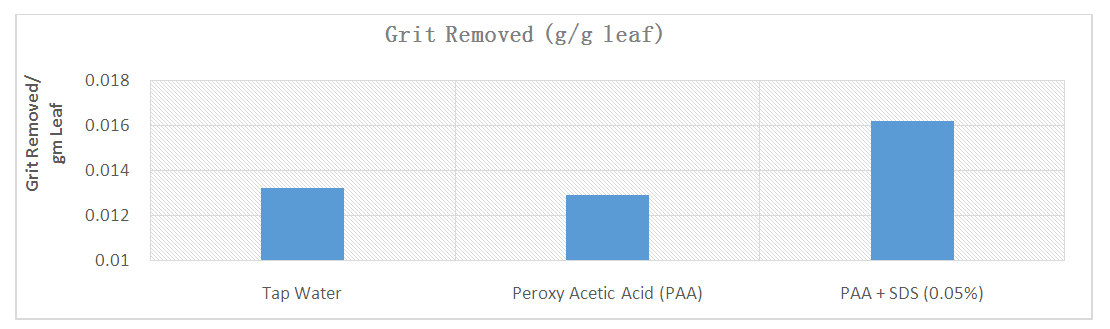
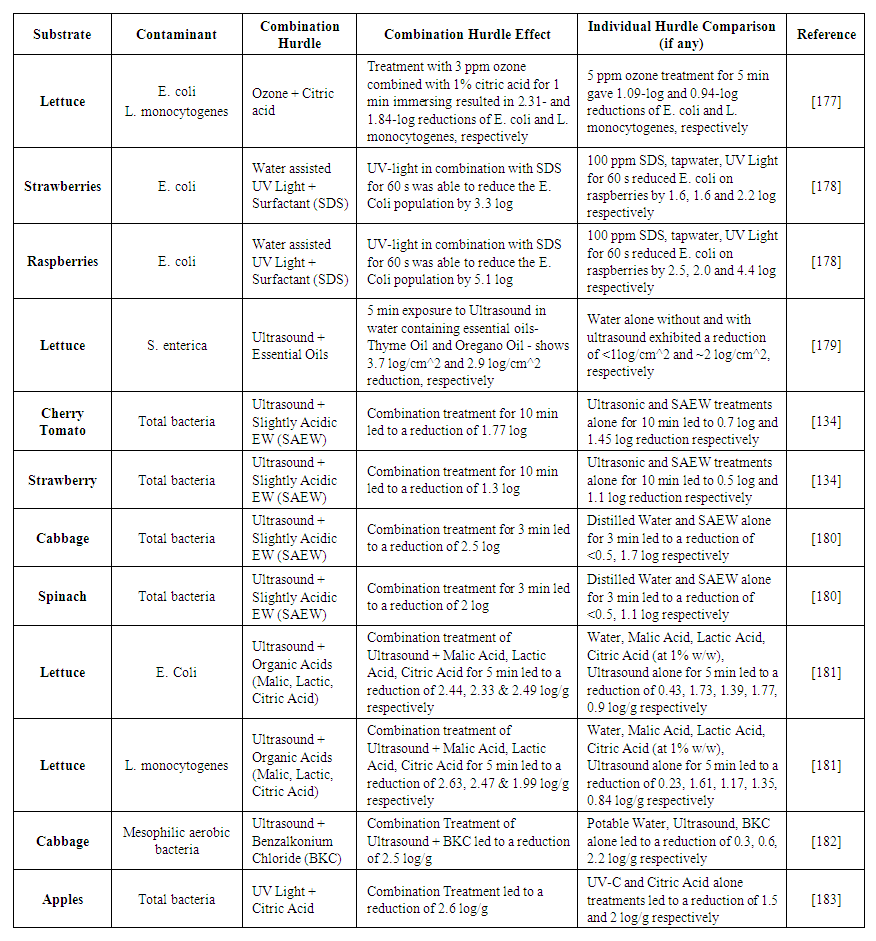
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML