-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2020; 10(1): 1-11
doi:10.5923/j.food.20201001.01

Anti-nutrients, Bioaccessibility and Mineral Balance of Cookies Produced from Processed Sesame Seed Flour Blends
Akusu O. M., Kiin-Kabari D. B., Isah E. M.
Department of Food Science and Technology, Rivers State University, Port Harcourt, Nigeria
Correspondence to: Akusu O. M., Department of Food Science and Technology, Rivers State University, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

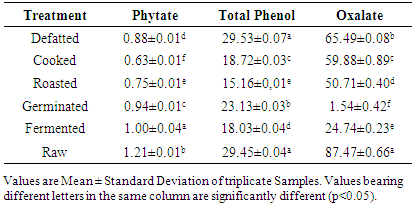
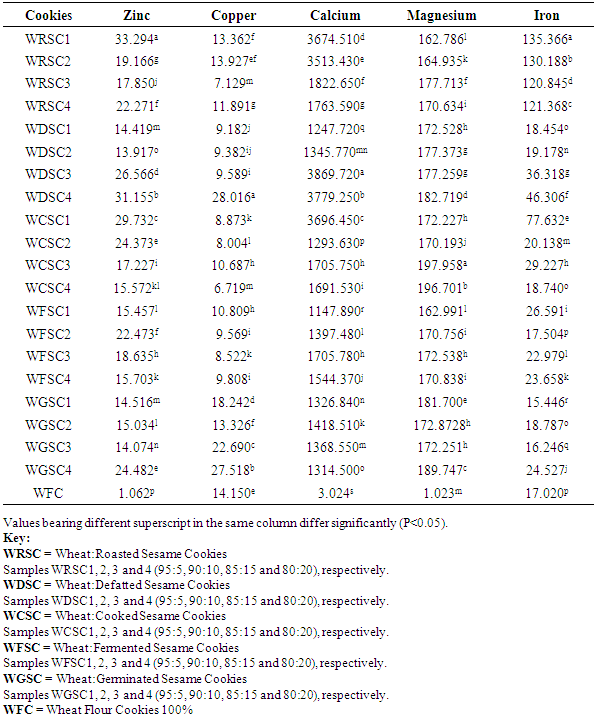
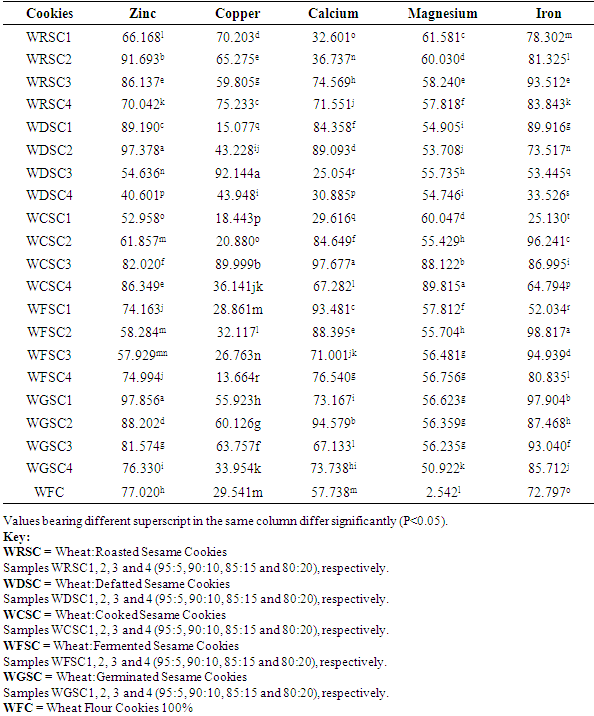
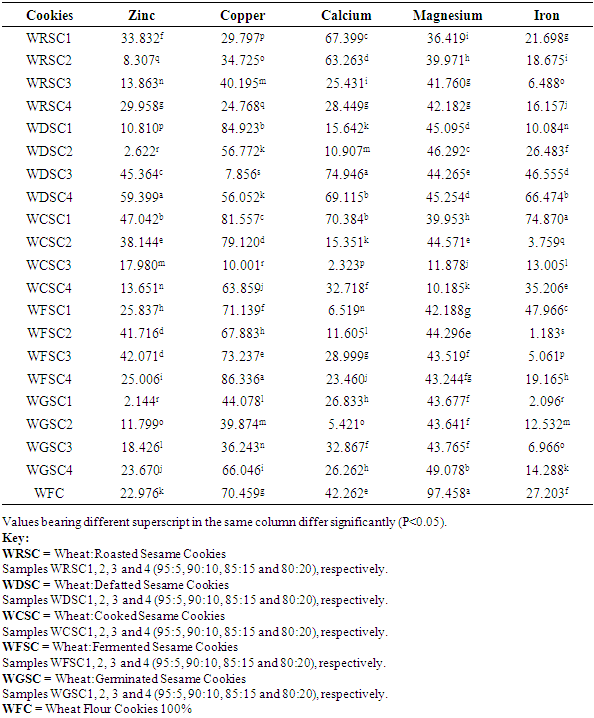
Sesame seed was processed into flour using five different processing methods such as defatting, cooking, roasting, germination and fermentation. The processed sesame seed flour was substituted to wheat flour at 4 levels (5, 10, 15 and 20%) with other ingredients to produce cookies. Effect of processing methods on anti-nutritional content of the flour blends, total mineral contents, in-vitro mineral bioaccessibility and mineral balance of the produced cookies were determined. Result revealed that raw sesame seeds had anti-nutrient values of 1.21mg/100g, 87.47mg/100g and 29.45mg/100g for phytate, oxalate and total phenol, respectively while processing methods revealed a significant reduction (P<0.05) in the values of these anti-nutrients that were analysed. In terms of the total mineral, calcium, magnesium and iron content of the cookies ranged from 2220.95 – 2916.9mg/100g, 257.219 – 278.587mg/100g and 30.573 – 45.550mg/100g, respectively. Percentage mineral bioaccessibility ranged from 40.60 – 97.856%, 15.077 – 92.144%, 25.054 – 97.677%, 2.542 – 89.815% and 25.13 – 98.817% for Zn, Cu, Ca, Mg and Fe, respectively. Bioaccessibility of minerals in the cookies samples increased significantly (P<0.05) with increased percentage substitution of cooked, roasted and fermented sesame seed flour samples. Cookies with 5% fermented sesame seed flour recorded high percentage bioaccessible zinc and magnesium of 97.856% and 97.907%, respectively. Mineral balance of the cookie samples ranged from 2.144 – 59.399%, 7.856 – 86.336%, 2.32 – 74.946%, 39.955 – 97.548 and 3.759 – 74.87% for Zn, Cu, Ca, Mg and Fe, respectively.
Keywords: Processing, Bioaccessibility, Mineral balance, Sesame seed, Cookies
Cite this paper: Akusu O. M., Kiin-Kabari D. B., Isah E. M., Anti-nutrients, Bioaccessibility and Mineral Balance of Cookies Produced from Processed Sesame Seed Flour Blends, International Journal of Food Science and Nutrition Engineering, Vol. 10 No. 1, 2020, pp. 1-11. doi: 10.5923/j.food.20201001.01.
Article Outline
1. Introduction
- Cookies are nutritive snacks obtained from single or composite dough which has been transformed into digestible and more appetizing product through the action of heat in the oven [1]. Cookies are classified based on the ingredient composition and processing techniques [2]. Due to increased demand for functional products, attempts are being made to improve the nutritive value and functionality of cookies by modifying their nutritive composition. This involves the use of non-wheat flour with attempt to increase the protein content and quality of the cookies and overcome the problems of high cost of wheat flour due to its importation to Nigeria and other countries whose climates are unfavourable for wheat cultivation. These limitations have prompted the search for available or underutilized crops, seeds and fruits with functional attributes to be incorporated as composite flours, fortifications and enrichments for the production of baked products [3]. As such, cookies as an easy to eat cherished snack has been produced from wheat/cashew apple residue as a source of fibre [4] and moringa leaf four has been used as protein fortification and enrichment in cookies production [5,6]. Few has been recorded on the usage of sesame seeds in the production of cookies despite its availability [7] and health benefits that has been reported by many researchers [8-10].Sesame seeds (Sesamum indicum) are tiny, flat oval seeds with a nutty taste. It is an important oil seed believed to have originated from tropical Africa with the greatest diversity [11]. Sesame seed is a staple food among many ethnic groups in Nigeria and it is cultivated in most areas of the middle belt and some northern states of Nigeria [7]. Sesame is an important source of oil (44-52.5%), protein (18-23.5%) and 13% of carbohydrate [9,10]. The seeds are consumed fresh, dried or blended with sugar. It is also used as a paste in some local soups. The meal is notable for its high protein concentration which is rich in methionine and tryptophan. Since these amino acids are missing from a number of other sources of vegetable proteins such as soybean. Thus, sesame seed flour can be added to recipes to give a better nutritional balance to healthy food products [12]. Apart from the mentioned amino acids, sesame seed is also rich in fat, carbohydrates, fibre and some minerals. The oil seed is renowned for its stability because it strongly resists oxidative rancidity even after long exposure to air [13]. The nutritional value of foods depends on their nutrient content and the bioavailability of these nutrients. Enhancement of the nutritional quality of sesame seeds can be anticipated through processing prior to consumption. Sesame is commercialized in a number of forms. Most sesame is processed directly into oil by the grower or within the producing region. It can also be sold in various processing stages for different uses such as meal, paste, confections and bakery products. Furthermore, sesame seeds can be consumed directly as a highly nutritious foodstuff [8]. However, lack of knowledge of the nutritional qualities of lesser known oil seed grown in developing countries like Nigeria is responsible for the poor utilization of these crops in different food formulations. Various processing technologies have helped in transforming food ingredients into healthier products with maximum nutritional value to ensure nutrient security of the population in developing countries [14]. Such techniques include cooking, roasting, defatting, fermentation and germination. These techniques play an important role in ensuring the food security of millions of people around the world, particularly the marginalised and vulnerable groups. This is achieved through improved food preservation, increasing the range of raw materials that can be used to produce edible food products and removing anti-nutritional factors to make food safe to eat [15]. Previous research work on oil based seeds highlight cooking, roasting, germination and fermentation of seeds as being more responsible for providing nutritionally better products than the raw seeds and the enzymes, especially α- amylase aid hydrolysis of the seed macromolecules [16,17] after the application of all these processing methods.Germination is a complex metabolic process during which the lipids, carbohydrates and stored proteins within the seeds are broken down in order to provide energy and amino acids necessary for the plant development [18]. Likewise, the metabolic changes that take place during different stages of roasting, fermentation and germination influence the bioaccessibility of essential nutrients [19]. Bioaccessibility of a nutrient is the fraction of ingested nutrient that is available for utilization in normal physical functions and storage [20]. Factors that influences its availability include chemical state of the nutrient, its release from food matrix, its interaction with other food components, presence of suppressor and other cofactors, formation of stable components that are easily metabolized [20]. Bioaccessibility of minerals in oil seed products is a less research area but the information is of high significant as oil seeds form major protein sources and is extensively used in complementary and supplementary foods, as well as in bakery products. Although exist numerous studies about the nutritional characteristics of sesame seeds, there is paucity of information on mineral bioaccesebility and mineral balance in value added products such as cookies formulated with roasted, cooked, defatted, fermented and germinated sesame seed flour. Mineral bioavailability can be affected by the presence of the anti-nutritional factors such as phaytate, tannins and polyphenols in foods [21]. The two most important techniques used to improve mineral bioavailability are reducing the phytate content in the foods or adding extra minerals in the fortification and blending process [22,23]. However, their nutritional quality is limited by the presence of anti-nutritional factors that exhibit undesirable physiological effects [24,25]. The anti-nutritional factors, being structurally different compounds are broadly divided into two categories: proteins (such as lectins and protease inhibitors) and others such as phytates, tannins, oligosaccharides, saponins, oxalates and alkaloids [26]. Phytate and oxalate usually found in the seed hulls lower the bioavailability of minerals and digestibility of plant proteins thereby limiting its use as food ingredient [27]. Anti-nutritional factors may also be classified according to their ability to withstand thermal processing, the most commonly employed treatment for destroying them. Heat labile factors include some components like protease inhibitors and lectins, whereas heat stable factors are represented by saponins, non-starch polysaccharides, antigenic proteins, estrogens and some phenolic compounds [28]. The negative impacts of the ingestion of anti-nutritional factors have extensively been reported. For instance, some factors, like trypsin inhibitor, affect protein utilization and digestion while others like phytic acid and tannins, affect mineral utilization [29,28]. Some anti-nutritional factors may exert beneficial health effects at low concentrations. Thus, the manipulation of processing conditions and removal or reduction of certain unwanted components of food may be required, to enhance nutritional quality [30,31]. The most common processing methods required to achieve this includes, cooking, roasting, germination and fermentation. Phytic acid, an anti-nutritional factor from raw sesame seed meal, could be reduced below detection limit by fermentation with lactic acid bacteria (Lactobacillus acidophilus) according to Mukhopadhyay [32]. Fermentation technologies play an important role in ensuring the food security of millions of people around the world, particularly the marginalised and vulnerable groups. This is achieved through improved food preservation, increasing the range of raw materials that can be used to produce edible food products and removing anti-nutritional factors to make food safe to eat [15]. Other processing methods, such as soaking, germination and cooking have been reported to improve the nutritional and functional properties of plant seeds [33,34]. This processing techniques can also reduce malnutrition by making micronutrients available for easy absorption; hence, increasing the utilization of sesame seeds. Industrial processing and utilization of sesame have not been fully developed in Nigeria as its utilization is restricted to producing regions. For most part, the surplus crop is commercialized, bulked and exported with minimal processing limited to cleaning and drying. Therefore, the objective of this study was to produce cookies from blends of roasted, cooked, defatted, fermented and germinated sesame seed flour in composite with wheat and to access the mineral bioavailability and mineral balance of the products, as well as the anti-nutrient properties of the processed flour, in view of increased utilization.
2. Materials and Methods
- Sesame seeds where purchased from an open market in Anyigba, kogi State. Wheat flour, sugar, margarine, salt, sodium bicarbonate, milk and vanilla flavour were purchased from confectionery store in Port Harcourt, Rivers State and transported in air tight high density polyethylene bag to the Food Chemistry Laboratory in the Department of Food Science and Technology, Rivers State University, Port Harcourt, Nigeria for processing and analysis. Digestive enzymes and bile salt were purchased from Sigma-Aldreich (Merck, Germany). All reagents and apparatus used in this study were obtained from the same Department and were of analytical grade.
2.1. Roasting
- The whole seeds of sesame seeds were roasted in an oven at 120°C for 1hr according to the method described by Mohamed et al. [35]. The samples were milled in a Braun (KMM 30, Bico, Chicago) mill to pass through a 0.5mm size sieve and stored in a sealed high density polyethylene bags until required for analysis.
2.2. Cooking
- Cooking was done using the method described by Makinde and Akinoso [31]. The whole sesame seeds were cooked at 100°C for 30min in the seed to water ratio of 1:10 (w/v). Consequently, the seeds were dried by hot air oven (model QUB 305010G, Gallenkamp, UK) at 40°C prior to milling and storage.
2.3. Defatting
- Sesame seeds were sorted, cleaned and milled into flour using Kenwood blender (Model A907D U.K). The flour was oven dried at 105°C for 1h to reduce the moisture content and to condition the fat molecules of the flour. The oil was extracted by solvent extraction with petroleum ether (b.p 40 – 60°C) in continuous soxhlet extraction apparatus for 3h to obtain defatted sesame seed flour. It was then sieved, packaged and set outside for analysis [36].
2.4. Fermentation
- The sesame seeds were dehulled, boiled in water for 6hr and cooled. The cooked seeds were placed in a plastic container with a tight lid and sealed. The samples were allowed to ferment at 35±2°C for 7 days and oven (model QUB 305010G, Gallenkamp, UK) dried at 105°C for 12hr to bring an end to fermentation, milled (Sieved with No 30 mesh) to obtain fermented sesame flour and stored in a glass container according to the method described by Akindahunsi [37].
2.5. Germination
- Sesame seeds were germinated as described by Okoli and Adeyemi [38]. The seeds were sorted to remove stones and other extraneous materials. It was thereafter soaked for 2hr to achieve hydration then rinsed, drained and spread thinly on jute sack for germination to take place. The germination process was closely monitored to prevent discontinuity of germination and mould growth which was achieved by constant wetting and intermittent uniform spreading of the germinating seedlings. Germination was carried out for three days. The germinated seedlings were thoroughly rinsed with water, drained, derooted, dried in a hot air oven (model QUB 305010G, Gallenkamp, UK) at 60°C for 6hr and then milled using a laboratory blender (KMM 30, Bico, Chicago) to pass through 0.5mm size sieve and stored in plastic bags until required for analysis.
2.6. Anti-nutritional Composition
2.6.1. Pythate Content
- The phytate content was determined using Hassan [39] standard method. Two grams of each finely ground sample was weighed into a 250ml conical flask and 100ml of 2% concentrated HCl was added. Allowed to stand for 3hr and filtered. After filtration, 50ml of the filtrate was pipetted into a 250ml beaker and 107ml of distilled water was added to improve acidity. Ten millimeter of 0.3% ammonium thiocynate solution was added as an indicator. The solution was titrated with standard iron III chloride (FeCl3) which contain 0.00195g iron/ml until a brownish yellow colour appeared and persisted for 5min. The phytic acid content was calculated using the formula below:
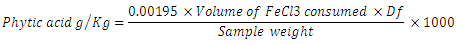
2.6.2. Total Phenol Content
- Total phenolic content (TPC) was determined colorimetrically using Folin-Ciocalteau reagent, as described by Singleton et al. [40]. Sample (0.4g) was extracted with 20ml of acidified methanol (1% HCl in methanol) for 1hr at 25°C, with vortex mixing at 5min intervals. Samples were centrifuged for 10min at 1200 rpm. Two replicate sample extract supernatants (0.5ml) was mixed with 2.5ml of Folin-Cicalteau reagent and allowed to stand at 25°C for 8min. Then 7.5ml of 20% sodium bicarbonate solution was added to the mixture. After 2hr at 25°C, absorbance was measured at 765 nm using a UV-visible spectrophotometer. A standard curve was prepared using various concentration of tannic acid and the results were reported as mg tannic acid equivalents/g of sample.
2.6.3. Oxalate Content
- Oxalate was determined following the AOAC [41] standard method. One gram of sample was weighed into 100ml conical flask. 75ml of 3M H2SO4 was added and the solution was carefully stirred intermittently with a magnetic stirrer for about 1hr and then filtered using Whatman No.1 filter paper. The sample filtrate (25ml extract) was collected and titrated against hot (80°C - 90°C) 0.1N KMnO4 solution to the point when a faint pink colour appeared that persisted for at least 30sec. The concentration of oxalate in each sample was obtained from the calculation: 1ml (0.1N) permanganate = 0.006303g oxalate.
2.7. Production of Cookies from Treated Sesame Seed Flours and Wheat Flour
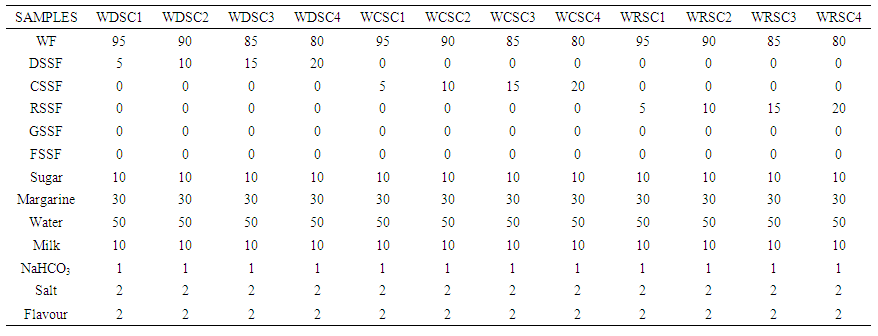 | Table 1A. Formulation of Cookie Recipe from the Blends of Wheat/Processed Sesame Seed Flours |
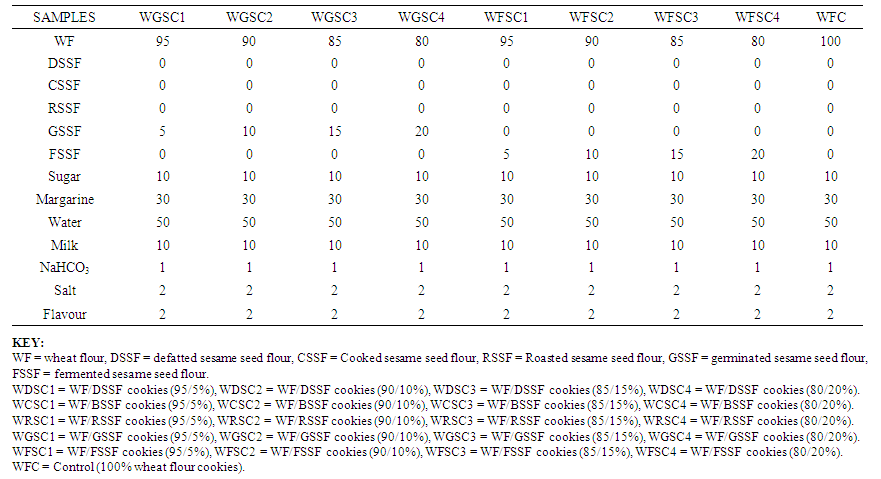 | Table 1B. Production Blends for Wheat/Processed Sesame Seed Flour Cookies |
2.8. In vitro Mineral Bioaccessibility
- In vitro mineral bioaccessibility was determined with stimulated peptic digestion, titratable acidity and pancreatic digestion method as described by Luten et al. [43]. A pepsin solution was prepared by dissolving 16g of pepsin (1:2,500) Cat No P7125 from porcine stomach mucosa) in 100ml of 0.1M HCl. The pancreatic solution contained 4g of pancreatin Cat No P1750 enzymes from porcine pancreas and 25g of bile extract B-8631, porcine in 1000ml of 0.1M NaHCO3.
2.8.1. Peptic Digestion
- The sample (10g) was mixed with 80ml of water in a 250ml conical flask. The pH was adjusted to 2.0 by adding 6M HCl and then 3ml of freshly prepared pepsin solution was added and the sample was made up to 100ml with distilled water. After mixing, the sample was incubated at 37°C in a shaking water bath for 2hr.
2.8.2. Titratable Acidity
- A homogenous aliquot of pepsin digest (20ml) was taken and 5ml of pancreatic mixture was added. The pH was adjusted to 7.5 with 0.5M NaOH. Titratable acidity was defined as the amount of 0.5M NaOH required in order to reach a pH of 7.5.
2.8.3. Pancreatic Digestion
- A homogenous pepsin digest aliquots (20ml) was weighed into a 250ml conical flask. Segments of dialysis tube containing 25ml of water and NaHCO3 were then added immediately to the amount of NaHCO3, being equivalent in moles to the NaOH used to determine the titratable acidity. After 30min, the pH attaining was 7.0 - 7.5, 5ml of the pancreatic mixture was added and the digest were incubated in a shaking water bath for 2hr at 37°C. The dialyzable fraction of mineral which represents the bioaccessible was quantitated by Atomic Absorption Spectrophotometry (atomic absorption spectrophotometer, Shimadzu AA6800, Tokyo, Japan).Bioaccessibilty (%) was calculated as follows:
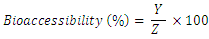 Where;Y = element content of bioaccessible fraction (mg mineral element /100g sample), which is the soluble mineral content.Z = total mineral content (mg mineral element /100g sample)Mineral balance = 100 – Bioaccessible mineral (%)
Where;Y = element content of bioaccessible fraction (mg mineral element /100g sample), which is the soluble mineral content.Z = total mineral content (mg mineral element /100g sample)Mineral balance = 100 – Bioaccessible mineral (%)2.9. Statistical Analysis
- All the analyses were carried out in duplicate samples. Data obtained were subjected to Analysis of Variance (ANOVA). Differences between means were evaluated using Tukey’s multiple comparison test, and significance accepted at P≤ 0.05 level. The statistical package in Minitab 16 computer program was used.
3. Results and Discussion
3.1. Anti-Nutritional Composition of Raw and Processed Sesame Seed Flour
|
3.2. Total Mineral Content of Cookies Produced from Wheat/Processed Sesame Seed Flour Blends
|
3.3. Mineral Bioaccessibility of Cookies Produced from Wheat/Processed Sesame Seed Flour Blends
|
3.4. Mineral Balance of Cookies Produced from Wheat/Processed Sesame Seed Flour Blends
|
4. Conclusions
- The study revealed that processing methods such as cooking, roasting, germination and fermentation significantly reduced the anti-nutritional components (phytate, oxalate and total phenol) of the flour blends. Total mineral content of cookies produced from processed sesame seed blends with wheat increased significantly than cookies produced from 100% wheat flour. Bioaccessibility of minerals in the cookie samples also increased significantly with increased percentage substitution of cooked and roasted sesame seed flour. Cookies with 5% fermented sesame seed flour substitution recorded highest percentage bioaccessible zinc and magnesium. Cookies with high mineral balance is an indication of low bioaccessibility of that specific mineral component.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML