-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2019; 9(3): 49-56
doi:10.5923/j.food.20190903.01
Received: Sep. 23, 2019; Accepted: Oct. 29, 2019; Published: Nov. 28, 2019

Physical, Nutritional Characteristics and Bioavailability of a Chili Pepper Cultivar (Capsicum annuum) Grown in Eastern Côte d'Ivoire
Ohouo Régina Antoinette Don , Pascal Amédée Ahi , Sophie Nadège Gnanguy , Lucien Patrice Kouame
Unité de Formation et de Recherche des Sciences et Technologies des Aliments, Université Nangui Abrogoua, Laboratoire de Biocatalyse et des Bioprocédés (LBB), 02 BP 801 Abidjan 02, Côte d’Ivoire
Correspondence to: Ohouo Régina Antoinette Don , Unité de Formation et de Recherche des Sciences et Technologies des Aliments, Université Nangui Abrogoua, Laboratoire de Biocatalyse et des Bioprocédés (LBB), 02 BP 801 Abidjan 02, Côte d’Ivoire.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The valorization of a chili pepper cultivar (Capsicum annuum) requires from the start information such as the physical and nutritional characteristics for a good exploitation of the fruits. The results indicate that the physical characteristics of the fruits of this chili cultivar are not influenced by the stage of ripening. The color of the fruits from the green stage to the red stage of ripening with the respective values of a * -0.33 to 1.68 for the ratio a * / b * in relation to the colors. The length of the fruit varies between (51.93 ± 0.78 cm and 31.5 ± 0.75 cm) and the width is between (1.69 ± 0.12 cm and 1.58 ± 0.19 cm) respectively for green and red fruits. At the nutritional level, the analyzed parameters showed a high moisture content (80.11 - 86.56%) in pepper fruits as well as a very low proportion of protein at all stages of ripening. The lipid content varies between 9.57% and 11.10% and the fiber content 2.19% to 2.74%. Subsequently, the molar ratios of phytate / calcium, phytate / iron, phytate / zinc, (phytate, calcium / zinc) and oxalate / calcium (Ox / ca) in the green pepper cultivar are 0.17, respectively 0.47; 5.96; 3.13-10-4 and 3.87. Unlike the Ox / ca molar ratio, the different molar ratios are below the recommended critical values. The nutritional composition of this chilli cultivar is leading to a new source of food ingredients.
Keywords: Nutritional Characteristics, Bioavailability, Chilli Cultivar, Ripening Stages
Cite this paper: Ohouo Régina Antoinette Don , Pascal Amédée Ahi , Sophie Nadège Gnanguy , Lucien Patrice Kouame , Physical, Nutritional Characteristics and Bioavailability of a Chili Pepper Cultivar (Capsicum annuum) Grown in Eastern Côte d'Ivoire, International Journal of Food Science and Nutrition Engineering, Vol. 9 No. 3, 2019, pp. 49-56. doi: 10.5923/j.food.20190903.01.
Article Outline
1. Introduction
- Chili is considered a spice, a fruit or vegetable belonging to the Solanaceae family [1,2]. It includes three domestic species of the genus Capsicum (C. annuum, C. frutescens and C. chinense) [3,4]. These species are native to the Americas where they have been cultivated for millennia. Chili (Capsicum annuum) is the most popular species in commerce and worldwide [5]. Peppers are widely used around the world for their nutritional composition [6]. Chili peppers contain anti-nutritional compounds that prevent the absorption of minerals. Indeed, anti-nutrients are chemicals that have been developed by plants for their own defense, among other biological functions they reduce the maximum use of nutrients (especially proteins, vitamins and minerals), thus preventing a optimal consumption of nutrients in a food and decreasing nutritional value [7]. However, chilli peppers can be consumed fresh or dried in the manufacture of pasta or sauces [8]. They are used as a natural colorant in food and cosmetics. Peppers have various medicinal properties [9,10,11] and they are also used in the manufacture of insecticides [12].As a result, factors such as cultivars and environmental conditions influence the nutritional components of chilies [13]. Previous work done by [14] showed that the essential oil of the chili cultivar (Capsicum annuum) which is the subject of our study is a good source of volatile compounds in particular, a strong presence in thymol and in limonene. These compounds with antioxidant properties have a beneficial effect on human health. Despite the many assets of chili pepper (Capsicum annuum), there are very few scientific studies related to the chili cultivar, subject of our study. It is therefore necessary to develop processes for valorizing the fruits of this pepper cultivar at different stages of ripening. Therefore, popularizing the fruits of this chili cultivar requires knowledge of the physical and nutritional properties of this pepper. In this study we determine the physical, nutritional and bioavailability characteristics of the chili cultivar (Capsicum annuum) grown in the eastern region of Côte d'Ivoire.
2. Material and Methods
2.1. Plant Material
- The fruits of the chili cultivar (Capsicum annuum) were harvested from a plot of Nangui Abrogoua University (Abidjan, Côte d’Ivoire).
2.2. Collection of Samples
- The fruits of the chili cultivar were grown in the period from February to June 2018. The plants and flowers were identified and authenticated at the Department of the National Center for Floristic Research (Félix Houphouët-Boigny University, Cocody-Abidjan). After harvest, the samples were placed in a polyethylene bag and immediately transported to the Centre Suisse de Recherches Scientifiques (Abidjan, Côte d’Ivoire) and stored in the dark at room temperature. The fruits of the chili cultivar were sorted according to each stage of ripening (green, yellow, orange and red). Then they were divided into two lots according to the coloring. One lot used for physical analysis and the other for chemical analysis.
2.3. Determination of Physical Parameters
- The average fruit size of the chili cultivar (70 fruits) at different stages of ripening, chosen at random, was determined by measuring length (L), width (w) and thickness (t) using Vernier calliper caliper, (Japan). To evaluate the specific gravity, the fruits were weighed using an electronic balance to the accuracy ± 0.001 (Pioneer-Ohaus, Switzerland). The shape has been described and evaluated in terms of the Caliber Index (CI) and aspect ratio (Ra) [15,16] according to the following expressions (1,2):
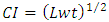 | (1) |
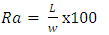 | (2) |
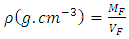 | (3) |
2.4. Approximate Analysis
- The various ripening stages of the chili cultivar were analyzed according to the [18] method. Moisture content was determined by drying (5 g) fresh sample in an oven (Solo Swiss, Switzerland) at 105°C to constant weight. The crude fiber content was estimated by weighting the insoluble residue obtained with the acid (H2SO4, 0.25 M) and the alkaline (NaOH, 0.3 M). The crude protein was estimated by the Kjeldahl method. It was calculated by multiplying the estimated nitrogen by 6.25. The lipid level was determined by hexane extraction in a Soxhlet apparatus and a rotary evaporator (B*U*CHI, Rotavapor R-300, Switzerland) allowed the solvent to be separated from the fat. Carbohydrate and energy value were calculated using the formulas described by Food and Agriculture Organization [19]. The results of the fibers, proteins, lipids, carbohydrates were expressed on the basis of the dry matter (DM). Vitamin C was determined by the titration method using 2,6-dichlorophenol indophenols as described in [18].
2.5. Analysis of Essential Minerals
- The mineral elements were analyzed after wet pickling under variable pressure SEM (SEM FEG Zeiss Supra 40PV) according to [20]. Approximately 10 mg of the ash residue sample was uniformly applied to a primed platform with double-sided adhesive carbon for analysis.
2.6. Anti-Nutritional Factor Analysis Oxalate Content
- The oxalate (Ox) assay was performed according to the method described by Day and Underwood (1986) using potassium permanganate. One (1) gram of oven-dried and ground sample was homogenized in 75 ml of 3 M sulfuric acid. The mixture was magnetically stirred for one hour and then filtered. Twenty-five (25) milliliters of filtrate were hot-titrated with 0.05M potassium permanganate solution until the steady pink turn. The oxalate content (TOx) was expressed according to the following formula (4):
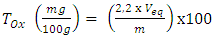 | (4) |
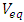 : Titrated volume
: Titrated volume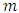 : mass of the sample
: mass of the sample2.7. Phytate Content
- Phytates were extracted from 4 g of the sample powder in 100 ml of chloridic acid (2%). The mixture was constantly stirred for 3 h and filtered. 5 ml of 0.3% ammonium thiocyanate (NH 4 SCN) was added to 25 ml of the filtrate. Fifty milliliters of distilled water were added to give the desired acidity. This solution was titrated with a ferric chloride solution at 0.00195 g / ml. The final solution was characterized by a persistent brownish yellow coloring. The phytate content (Tphy) was calculated from the equation according to the following formula (5):
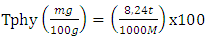 | (5) |
2.8. Bioavailability of Minerals Content
- The molar ratios of phytate: calcium (Phy / Ca), phytate: iron (Phy / Fe); phytate: Zinc (Phy / Zn); phytate, calcium: zinc (Phy. Ca / Zn) and oxalate: calcium (Ox / Ca) were calculated to estimate the relative bioavailability of calcium, zinc and iron in the presence of antinutrients. Molar ratios were calculated using the following mathematical formula (6):
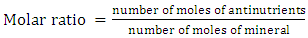 | (6) |
2.9. Statistical Analysis
- The statistical analysis was performed using the XLSTAT 2014 software. The data obtained (mean ± standard deviation) were submitted to the Duncan test to evaluate the differences between the P <0.05 means. All experiments were conducted in triplicate.
3. Results
3.1. Physical Characteristics of Chilli Peppers at Different Stages of Ripening
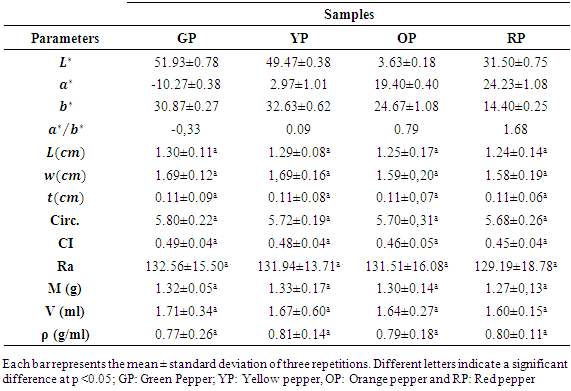
- The results of the physical characteristics are recorded in Table 1. Indeed, the L * values specify the brightness or luminosity of the fruits of the peppers. This value ranges from 31.5 ± 0.75 for red fruits to 51.93 ± 0.78 for green fruits. The values of a * are positive in yellow, orange and red fruits of 2.97 ± 1.01, 19.4 ± 0.40 and 24.23 ± 1.08, respectively. While the value of a * is negative in green fruits with an average value of -10.27 ± 0.38. The ratio a * / b * determines the colorations from green to red with the respective values of -0.33; 0.09; 0.79 and 1.68. However, peppers have a length that varies considerably between (1.30 ± 0.11) cm and (1.24 ± 0.14) cm respectively for green and red fruits. The average width of the fruits varies between (1,69 ± 0,12) cm and (1,58 ± 0,19) cm. However, the thickness (t) of the fruits remains unchanged 0.11cm for all fruits. The circumference (Cir.), Calyx index (CI) and aspect ratio (Ra) vary very little from one fruit to another. The average mass of pepper fruits varies very little from green (1.32 ± 0.05) cm to red (1.27 ± 0.13) cm. It is the same for the volume with the average values ranging from (1.71 ± 0.34) cm for the green fruits to (1.60 ± 0.15) cm for the red fruits. In addition, the average value of the density of the fruits varies between (0.77 ± 0.26) cm for the green fruits and (0.80 ± 0.11) cm for the red fruits. The statistical analysis of the morphological parameters the fruits of the chilli cultivar showed that the stage of ripening has no impact on the various parameters measured.
|
3.2. Approximate Analysis
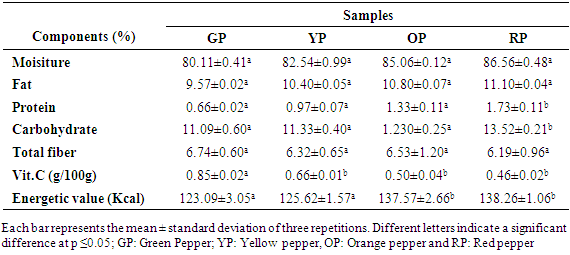
- The nutrient components of the chili cultivar studied are described in Table 2. Indeed, the humidity levels of fresh chilies range from 80.11% to 86.56% of the fresh weight respectively for fruits in the green to red stage. In addition, the lipid contents differed from 9.57% to 11.10% DM. In contrast, protein levels are relatively low in chili pepper fruits (Capsicum annuum) with a slight increase (0.66 - 1.73%) DM from green to red. However, carbohydrate contents vary from (11.59-14.30%). In addition, the total fiber contents vary from 2.74-2.19% DM. Vitamin C content decreases considerably from fresh green to red fruits with average values of 0.85g / 100g to 0.46g / 100g respectively. Unlike the vitamin C content, the results showed that maturity did not significantly (p ≥ 0.05) influenced nutrients in the fruits of the chilli cultivar (Capsicum annuum).
|
3.3. Essential Minerals
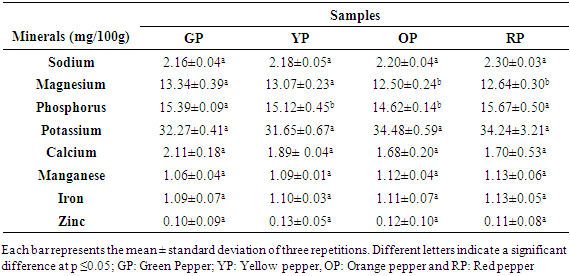
- Eight essential minerals were present in the samples at different grades (Table 3). Indeed, these minerals varied from green to red stages. In addition, the predominant mineral was potassium with average values ranging from 31.65 mg / 100g to 34.48 mg / 100g DM. No difference was observed in minerals (p ≥0.05) during ripening.
|
3.4. Antinutritional Factors
- In general, phytates and oxalic acid are present in the chili cultivar during ripening stages (Fig.1). In fact, oxalate levels in the chili cultivar remained virtually unchanged from green, yellow, orange and red stage stages with values 26.06 mg / 100g, 25.03mg / 100g, 26.85 mg / 100g and 26.91 mg / 100g respectively.
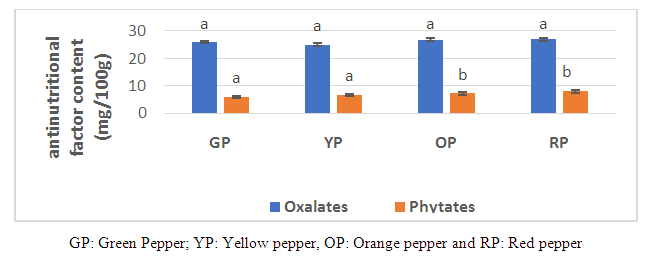 | Figure 1. Anti-nutritional factor composition of chili pepper cultivar at different stages of ripening |
3.5. Molar Ratio: Anti-Nutrient / Nutrient
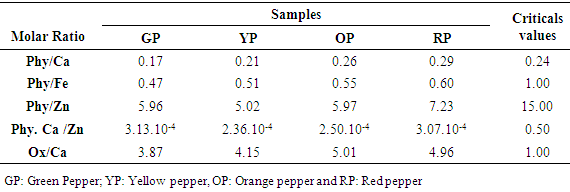
- Table 4 shows the molar ratios of phytate / calcium, iron, zinc; phytate. calcium / zinc and oxalate / zinc with their recommended critical values. The molar ratios of phytate / calcium, iron, zinc; phytate. calcium / zinc and oxalate / ca (ox / ca) in the green chili pepper cultivar were 0.17; 0.47; 5.96; 3.1310-4 and 3.87. In contrast to the ox / ca molar ratio, these different calculated ratios were lower than the critical values.
|
4. Discussion
- The fruits of the chili pepper cultivar (Capsicum annuum) change from green to yellow then to orange and finally to red during ripening. These different colorations are explained by the presence of chlorophyll in the green fruits and the accumulation of carotenoids in the chromoplasts during fruit ripening. The evolution of the color from green to red would be due to the change of pigments during fruit growth and maturity. In fact, chlorophyll and carotenoids are pigments responsible for the coloring green of red fruits [21]. These compounds are widely used in the food industry as a natural colorant [22,4]. Thus, these compounds from the fruits of the chili cultivar could be used for the production of natural colorant in the food industry. The fruits of the chili cultivar (Capsicum annuum) are long and broad with relatively different average values. In fact, assessing average fruit size is important for meeting quality standards, increasing market value, monitoring fruit growth and sorting fruit. Moreover, our results are consistent with earlier results that indicate that size varies with fruit ripening conditions [23]. In addition, the assessment of the weight of chili pepper aims to determine the amount of material in a fruit. Indeed, this mass is important to the extent that it allows to express the nutritional value of a food for humans. Volume is also an important performance trait in horticultural crops [24]. It should be noted that the ripening stage has no influence on the morphological characteristics of the fruits of the pepper cultivar studied. Our results corroborate those of [1] which state that plant morphological variation and fruit shape, as well as fruit color, strongly depend on the cultivated chili varieties. Regarding the results of the nutritive values, they show that the studied pepper cultivar at very high moisture levels at different stages of ripening. In general, the water content in fresh peppers at these different stages remains very high. Indeed, the moisture content of any food is an index of its water activity. This property explains why peppers are eaten within 72 hours or 96 hours of harvesting. Moisture is therefore a limiting factor in the preservation of peppers. Because, it promotes the proliferation of micro-organisms capable of altering the food. Thus, the obtained result for green pepper is comparable to that reported by [25] which is 81.9% of variety S49 of green chili (Capsicum annuum) grown in Anand (Gujarat, India). On the other hand, the protein content remains very low for this studied cultivar. Therefore, protein intakes of other food components in a spice-rich diet will improve protein content. These results are similar to studies conducted by [26]. The presence of raw fibers contributes to the maintenance of human health because they stimulate the contraction of the intestine and promote bacterial activity in the colon. A number of studies reported by [27]; have reported that the dietary fiber content is related not only to the quantitative aspect, but also to the qualitative aspect because they are associated with polysaccharides with antioxidant activity. With regard to, the relatively low lipid contents of the fruit samples confirm the conclusions of many authors who have shown that vegetables are not excellent sources of fat [28]. The estimated calorific values (123.09 ± 3.05) Kcal / 100g DM to (138.26 ± 1.06) Kcal / 100g DM correspond to the general observation that vegetables have a low energy value because of their low raw fat content and their relatively high humidity [29]. Regarding Vitamin C helps to strengthen the immune system. In fact, the vitamin C contents were higher at the green stage of ripening, moreover these contents decreased until the red stage. This is because the stage of ripening and variations in growing conditions and the genetic parameters of the fruits also affect the level of ascorbic acid in them [30]. Therefore, these results differ from those of [31], which states that in fruits of peppers, the level of ascorbic acid increases as the fruit matures and this higher level is noticed in the red stage fruit. This Capsicum chili cultivar has a high vitamin C content that is able to meet the recommended daily dose of 60 mg per 100 g of raw pepper according to [32]. It is therefore advisable to consume this cultivated chilli cultivar to enjoy at best vitamin C because it is degradable under the effect of heat. The chilli cultivar studied at different stages of maturation is a good source of minerals because potassium, phosphorus, magnesium was mostly present. In fact, sodium, calcium, manganese, iron and zinc were in the minority. In addition, potassium was the predominant mineral in this chili pepper cultivar at each ripening stage. These results are similar to those of [33]. In addition, the decrease or increase of some minerals in maturation stages is explained by the fact that the same plant species may have a different response to the absorption of nutrients from the soil even when they have been cultivated under the same conditions [34]. These results then confirm those of [35] who claim that nutrient compounds vary from one stage to another because of climatic conditions, variety of cultivars and differences in analytical procedures. Thus, potassium is of great importance for humans because it acts inside cells in combination with sodium. It participates in cardiovascular health when it is balanced [36]. In addition, the nutritional composition of the fruits of this chili cultivar is important because it is closely related to the quality and preservation of the products. The presence of anti-nutritional substances could be detected in pepper fruits at the different stages of ripening studied. Indeed, oxalic acid and phytate are present in many plants. However, the level of oxalic acid varies from one cultivar to another, taking into account growth and distribution conditions in the plant [37]. On the other hand, the oxalate levels of the green pepper cultivar at the red maturation stage are similar to the studies conducted by [33] that obtained values ranging from 20.7 to 48.2 mg / 100 g. Phytate content remains low at all ripening stages. Thus, these results differed from those of [38], which did not detected traces of phytate in three cultivars of capsicum annuum grown in Ethiopia. Although phytate and oxalic acid are present in this chili pepper their mineral inhibitory effect can be reduced by soaking and boiling, which reduces their content in foods. However, the antinutritional elements would be the object of new research because of their antioxidant activity being able to have an anti-cancerous property [39] and some authors would recommend to limit their degradation during the technological processes [40]. Thus, this anti-cancer activity is due to their ability to chelate minerals that are essential cofactors of many oxidoreductase reactions. With regard to the bioavailability of minerals, the anti-nutrient / mineral molar ratios of the chili cultivar at different stages of ripening have yielded interesting results. Indeed, the molar ratios of Phy / Calcium; phytate / iron; phytate / zinc and (phytate, calcium / zinc) are below the respective critical values 0.24, 1.00, 15.00 and 0.5 recommended. These results indicate that the bioavailability of Ca, Fe and Zn is not influenced by the phytate contained in the fruits of the chili cultivar (Capsicum annuun) during ripening stages. While the molar ratio Ox / Ca is greater than the value 1.00 recommended. This explains why the bioavailability of Ca is inhibited by the presence of oxalate. Thus, the presence of oxalate in the chili cultivar will reduce calcium absorption. It should be noted that because of their poor absorption or lack of food, minerals such as calcium, iron, zinc are considered a deficiency in the diet [41]. Hence their importance in human nutrition. According to [42], oxalate is a potent dietary calcium inhibitor while phytic acid has a much lower inhibitory effect. These results show that the absorption of Fe, Ca and Zinc have no influence on phytate. Thus, regular consumption of this chili cultivar fruits will prevent iron and zinc deficiencies. Indeed, [43] reported that the bioavailability of iron in chili pepper was increased by cooking. The author [44] reported that zinc could participate in the regulation of blood pressure and the pathogenesis of hypertension. However, the absorption of iron facilitates the transport of hemoglobin in the human body. According to [45] the absorption of iron, calcium and zinc may be affected by a reduction in gastric acid secretion (e.g., from mucosal atrophy from protein energy malnutrition or bacterial‐induced gastritis [46]. However, it is well known that capsaicin present in the chili pepper increases the secretion of stomach acid and therefore promotes the absorption of iron, calcium and zinc [47]. In view of these molar ratios, minerals such as iron, zinc and calcium would be bioavailable because of the capsaicin contained in this pepper cultivar at different stages of ripening.
5. Conclusions
- This study highlighted the physical, nutrient and bioavailability characteristics of a pepper cultivar (Capsicum annuum) cultivated in Côte d'Ivoire. Thus, the physical characteristics revealed the presence of certain substances such as carotenoids in the red fruits of the chilli cultivar. These compounds with antioxidant properties contribute to human health and are also used as a natural dye in the food industry. In addition, the nutritional characteristics and bioavailability of the nutrients in these fruits showed the presence of various nutrients at all stages of ripening. Based on the results of this study, the fruits of chilli cultivar (Capsicum annuum) regardless of their stage of ripening are rich in various nutrients. The valorization of this cultivar of pepper is very important. While there is currently a lot of international interest in developing the potential of chili pepper production in Africa, the role of chili peppers in this cultivar in the local diet should be taken into account by development programs. In addition, these fruits of the chilli cultivar lead to a new source of food ingredients.
ACKNOWLEDGEMENTS
- The authors wish to thank the Centre Suisse de Recherches Scientifiques (CSRS) for the financial and technical supports of the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML