-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2019; 9(2): 45-48
doi:10.5923/j.food.20190902.03

Probiotication of Fruit Juices by Supplemented Culture of lactobacillus acidophilus
Kidist Fikre Worku, Henok Kurabachew, Yassin Hassen
School of Nutrition, Food Science and Technology, Hawassa University, Ethiopia
Correspondence to: Kidist Fikre Worku, School of Nutrition, Food Science and Technology, Hawassa University, Ethiopia.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The beneficial effects of food with added live microbes (probiotics) on human health are being increasingly promoted by health professionals. probiotic was technically defined by an Expert Committee as ‘‘live microorganisms which upon ingestion in certain numbers exert health benefits beyond inherent general nutrition’’. Fruit juices are also an alternative vehicle for the incorporation of probiotics because they are rich in nutrients and do not contain starter cultures that compete for nutrients with probiotics. Three types of fruit was used in this research and five sugar type (table sugar brix 11, 15, Glucose, Fructose and Sucrose) and all of them were determined for microbial count, pH, titratable acidity and total soluble solid. All samples also stored for six days and viable bacterial count, pH and titratable acidity were done at every three days. The result showed that during probioticaion pH of all three samples were reduced while titratable acidity was boosted with increasing incubation time.
Keywords: Probiotics, Fruit juices, Microbial counts, CFU
Cite this paper: Kidist Fikre Worku, Henok Kurabachew, Yassin Hassen, Probiotication of Fruit Juices by Supplemented Culture of lactobacillus acidophilus, International Journal of Food Science and Nutrition Engineering, Vol. 9 No. 2, 2019, pp. 45-48. doi: 10.5923/j.food.20190902.03.
Article Outline
1. Introduction
- Fruit juices are essential components of the human diet. Apart from being good sources of vitamins, minerals, and fiber, these foods are also a rich source of potentially bioactive compounds (Palafox-Carlos, et al., 2011). Fruit juices contain high amounts of sugars which could encourage probiotic growth. Nowadays, healthy foods mean “functional foods”, and generally functional food is the food that exerts beneficial effects or more specific body functions, in addition to the traditional nutritional effects. Well-known examples of functional foods are those containing or prepared with bioactive compounds, such as dietary fiber, oligosaccharides, and active “friendly” bacteria that promote the equilibrium of intestinal bacterial strains. In addition to the well-established functional ingredients, such as vitamins, minerals, and micronutrients, probiotics belong to an emerging generation of active ingredients, which includes prebiotics, phytonutrients, and lipids (Jankovic, et. al., 2010). Functional foods represent one of the most interesting areas of research and innovation in the food field, as suggested by the increasing number of scientific papers dealing with this topic since 2007. The market of functional foods is characterized by an increasing trend, some researchers reported that probiotic foods represent ca. 60%–70% of functional foods (Tripathi and Giri, 2014). Generally, the concentrations of 106 and 107-108cfu mL-1 (cfu g-1), respectively, have been accepted as the minimum and satisfactory levels. (Karimi, et al., 2011)Lactobacillus and Bifidobacterium (Genus of Lactic acid bacteria) are most commonly used probiotics in food and feed. Other microorganisms such as yeast; Saccharomyces cerevisiae and some Escherichia coli and Bacillus species are also used as probiotics. Probiotics have been used for centuries in fermented dairy products. However, the potential applications of probiotics in nondairy food products and agriculture have not received formal recognition. In Ethiopia there is very few research were done on probiotics like Shukla, et al., (2013) on Development of Probiotic Beverage from Whey and Pineapple Juice and reported that good quality probiotic beverage with therapeutic value prepared by using a 65:35 blend of whey and pineapple juice inoculated with 1 percent inoculums of Lactobacillus acidophilus. But this study focuses on developing probioic fruit juice without using any milk/ milk products.
2. Materials and Methods
- Sample Collection and preparation of fruits juicesSample of well ripened locally available fruit (Orange, Banana and Apple) were purchased from local farms (Apple from Arbaminch, Chinka, Orange from Yelagnaw agro industry, banana Arbaminche). After collection of the samples, the fruits were washed with tap water followed by sterile water to remove foreign matters. The fruits are carried on the screening conveyor to take away diseased, green, old and damaged oranges, banana and apple then washed, Peeled, cutting in to small pieces, extracting juices, filtered and finally packed and stored under refrigerator at 4°C till analyzed (Kumar et al., 2013).Inoculation of Lactobacillus acidophilusThe Lactobacillus acidophilus bacteria in the frozen cultures was activated by MRS broth then incubated for 48 h at 32°C then from growth of bacteria in the broth loop full of sample was taken and spread plated on Man Rogosa Sharpe (MRS) agar, after incubation anaerobically for 48 hrs at 37°C. A 1% inoculum was added, incubated at 37°C for 12-14 h, collected by centrifugation at 8,000xg for 15 min at 0 C and washed twice with 0.1% peptone water and inoculated in the pasteurized fruit juice. (Mustafa, et. al., 2016, King and Su, 1993). Then, 0.33 grams of Lactobacillus acidophilus was directly added to all bottles. All of the samples were incubated at 38°C for 36 hours and the acidity and pH test was done. The samples were refrigerated at 4°C. The produced probiotic juices were analyzed directly for counting the microbes (Marhamatizadeh, 2009).Producing Products with Apple, Orange and Banana Concentrate with Lactobacillus Acidophilus Bacteria by Adding Glucose, fructose and sucrose.The only difference of this step was adding 30% of Glucose, fructose and sucrose.Analytical methodsTotal soluble solidsSoluble solid was measured by Refractometer Brix by methods of (Board, 1988). The pH of the samples were measured using pH meter which was first standardized using buffers of neutral values (7.0 pH), acidic (4.0 pH) and basic (9.0 pH) at 25°C (Merothet al., 2003). Titratable acidityoutlined by (Board, 1988).Measuring the Shelf Life Periods of the Products:For this purpose, 1000 grams of all produced compounds was refrigerated for 6 days and their shelf life was measured at every 3 days. The pH, titratable acidity and microbial count of all the samples were measured (Mohammad, et al., 2012).Experimental DesignA factorial design was used to derive mathematical models for describing the effects of the independent variables on the dependent variables (Myers et al., 2009). The three independent variables were sugar type (Table sugar (with two degree brix 11 and 15), Glucose, Fructose and Sucrose), fruit type (Apple, Orange and banana) and storage days (Day 1, 3 and 6). And the dependent variables determined were microbial count, pH, Titratable acidity, total soluble solid. Each experiment was repeated three times with total of 135 experiments. Statistical analysisStatistical procedures as described by (Snedecor and Cochron, 1977) was used to analyze the data for the interpretation of results. Mean, standard deviation and analysis of Microsoft Excel software and variance (ANOVA) was used to describe the results. Differences at p < 0.05 were considered as significant.
3. Results
|
|
|
|
|
4. Discussion
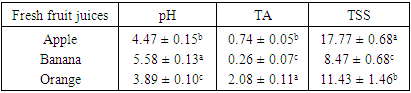
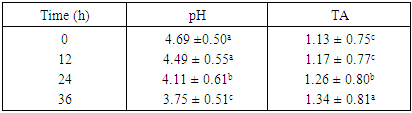
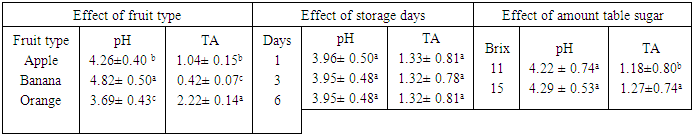
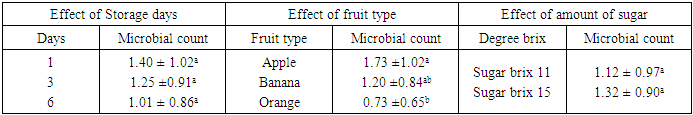
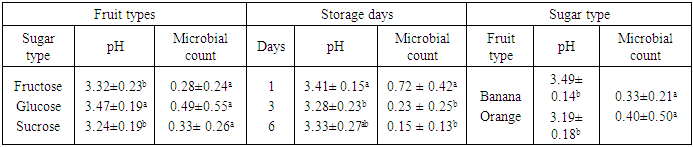
- The result obtained from physico-chemical properties of the fresh fruit juices with probiotication were shown as follows. Physico-chemical properties of the freshly prepared fruit juices were of the following values, the pH were 4.47, 3.89 and 5.58 for Apple, Orange, and Banana, respectively, with percentage titrable acidity (as citric acid) range of 0.74g/100ml, 2.08g/100m1 and 0.26 g/100ml. And total soluble solid (%) ranged from 17.2, 11.43 and 8.47 respectively (Table 1). This result is in accordance to the work done by (Kumar et al. 2009) on physico-chemical analysis of fresh fruit juices.A rapid decrease in pH in the beginning had a great importance for the quality of the end product (Table 2) (Vianderet al., 2003). The rapid increase in acidity minimizes the influence of spoilage bacteria. In the slowly acidified medium, lactic acid fermentation can be suppressed by butyric bacteria activity (Table 2) Karovicová and Kohajdová, 2003).The changes in pH and acidity during the growth of probiotic bacteria in different fruit juices were also measured (Table 2) the pH of all three juices with probiotic bacteria showed gradual reduction. Zero hour pH was recorded as 4.69. As incubation time increased it lowered the pH as low as 3.75, after 36 hours. This finding agreed with (Mohan 2013) which study on probiotication of fruit juices by lactobacillus acidophilus. This finding is also in agreement with (Mohammad, et, al., 2012) which stated that pH was reduced from 3.8 to 2.5 during production time.In the contrary the amount of titratable acidity obtained during bacterial growth was increased, at zero hour titratable acidity was 1.13 and after 36 hours of incubation titratable acidity raised to 1.34.Significant drop in the initial pH particularly after 12 hours incubation periods of the probioticated juices was observed. The lactic acid culture (L. casei) actively fermented the fruit juices and lowered the pH especially Banana pH was dropped more than the rest two juices (Apple and Orange). However, this result agrees with the of Mohan et al., (2013), who studied the probiotication of tomato juice by lactic acid bacteria and found out that the lactic acid cultures reduced the pH to 4.1 or below and increased the acidity to 0.65% or higher. The amount of titratable acidity also showed significant difference it increases as incubation goes up from zero to 36 hours. In the production of all probioticated fruit juice there was significant different on pH. Banana had the highest pH, followed by Apple and Orange. The result also showed that there was also significant different in titratable acidity, the highest acidity amount was recorded for Orange followed by Apple and the least acid amount was obtained from banana sample (Table 3).The effect of degree brix 11 and 15 was not significant on pH but it showed that it does have a little significance on titratable acidity (Table 3). And in the production of probiotic bacteria on juices degree brix 11 and 15 have significant different on both pH and titratable acidity. Brix 11 was the most appropriate situation, with respect to the acidity and pH, for probiotic bacteria growth. But the result was no statistical difference. The result was agreed with the work of Mohammad, et, al., 2012) A good probiotics has to tolerate low pH in order to survive HCl in stomach (Corcoran, et al., 2005). According to the result obtained from (table 4) microbial counts (growth of Lactobaccilus acidophilus) was reduced from 1.40 x 1010 to 1.01 x 1010 for counts of day one and day six respectively but there is no statistical difference in microbial count. Similar result was observed in storage of probioticated fruit juices with the three sugar type (glucose, fructose and sucrose). Microbial growth reduced from 0.72 to 0.15 for day one and day six, there was significant different on growth of day one with day three and six (Table 5). The reduction of microbes might be due to environmental stress or rising of internal acidity. Based on the effect of different fruit type the observed probiotic seems to grow on Apple more than Orange and Banana. There was no significant difference between bacterial growth in Orange and Banana and Banana and Apple but there was a significant different on microbial growth on Apple when compared to the rest (Table 4). Apple juice was found to be better fermented product than the other substrates which might probably due to higher moisture and sugar content (Mohan, et al., 2013).The effect of sugar type on microbial growth indicated that glucose had better growing then Fructose and Sucrose but statistically there was no significant difference (Table 5). No significant differences were observed to viability of cells during drying in the presence or absence of glucose (or fructose,…) however, survival during storage was higher in their presence for all growth media tested (Carvalho, et al., 2003). With the three sugar type bacterial growth on banana and Orange were almost the same and had no significant different.To be more useful in the body, the living probiotic bacteria number should be at least greater then 107 CFU per gram. In this research, by “direct microscopic count” method, the number of probiotic bacteria was estimated above 108 to 1010 (Table 4 and 5). So the products were nutritious and useful enough. The Results indicated that the juices served as a good medium for growing Probiotics. According to the Codex Alimentarius standard, a commercial probiotic beverage should possess a minimum viable count of 106 CFU/ml at the time of consumption (David, et al., 2013).
5. Conclusions
- Fruits are reported to contain a wide variety of antioxidant components, including phytochemicals. Phytochemicals, such as phenolic compounds, are considered beneficial for human health, decreasing the risk of degenerative diseases by reduction of oxidative stress and inhibition of macromolecular oxidation. With all this importance fruits also incorporated with probiotics because they serve as a healthy alternative for dairy probiotics, cholesterol free and also favor consumption by lactose intolerant consumers. All fruit juice (Apple, Orange and Banana) used to grow probiotic bacteria Lactobaccilusacicdophilous were a good medium. Table sugar brix 11 and 15 was used on this finding indicated that degree brix 11 was better than brix 15. In all other sugar (Glucose, Fructose and Sucroes) probiotic bacteria were grow on it insignificantly. In the storage of Probioticated fruit juice probiotic bacterial was reduced gradually.
ACKNOWLEDGEMENTS
- The authors would like to thank Hawassa University, Research and Technology Transfer Vice-president Office, and College of Agriculture, Graduate Studies and Research Coordination Office for their financial support to conduct this research. Moreover, the authors would also like to thank the food science laboratory technicians and Mr. Zemenu kere and Esayas Kinfe for their technical support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML