-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2018; 8(6): 131-141
doi:10.5923/j.food.20180806.01

Nutritional Evaluation of Commonly Used Local Weaning Food Processed and Sold in the Mount Cameroon Region
Asoba Gillian Nkeudem1, 2, Sumbele Irene Ule Ngole2, Anchang-Kimbi Judith2, Teh Rene Ning2, Samuel Metuge1, Kaptso Kuaté Giscard1, 3
1Department of Social Economy and Family Management, Higher Technical Teachers' Training College, University of Buea, Kumba, Cameroon
2Department of Zoology and Animal Physiology, Faculty of Science, University of Buea, Buea, Cameroon
3Chemical Engineering and Mineral Industries School (EGCIM), University of Ngaoundere, Ngaoundere, Cameroon
Correspondence to: Kaptso Kuaté Giscard, Department of Social Economy and Family Management, Higher Technical Teachers' Training College, University of Buea, Kumba, Cameroon.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Malnutrition is one of the major world health problems facing developing countries. Weaning includes the long critical period (the age of 5–6 months), when the child slowly gets used to other adults' food. Death and morbidity due to Protein Energy Malnutrition in infancy continue to scourge populations in many parts of the third world. This study was carried out to assess the quality of some local weaning food processed and sold in the Mount Cameroon region. Samples used required collecting of some local weaning food namely: Vita Force (VT), Soytine (ST), Soyaconia (SC), Soya Pap (SP), Tanty Reine (TR), Dina Baby (DB) and Cerelac® biscuit produced by Nestlé©. Proximate composition, sugars and fatty acids profile of local weaning food were carry out using the AOAC methods, HPLC and GLC-FID analysis. Mineral content using the Atomic Absorption Spectrophotometer and Amino acid, Vitamin B determined by HPLC analysis as well as Ascorbic Acid and fat-soluble Vitamin. Microbiological analysis were done using Coliform count and total viable count methods. All data obtained was statistical analysed using Duncan’s multiple range test. Results obtained showed that macronutrient composition, energy value and moisture contents of local weaning food are significantly different (P < 0.05). Mineral composition showed that calcium contents range from 230.48 mg.100g-1 (VF) to 322.30 mg.100g-1 (TR), phosphorus from 230.48 mg.100g-1 (VF) to 322.302 mg.100g-1 (TR), zinc from 1.91 mg.100g-1 (DB) mg.100g-1 to 2.45 mg.100g-1 (TR); and iron content varied from 17.32 mg.100g-1 (DB) to 21.08 mg.100g-1 (VF). In all local WF samples, octadecenoic (oleic) acid which is a mono unsaturated fatty acid had the highest value close to 3.50% in all samples, followed by octadecadienoic (linoleic) acid a polyunsaturated fatty acid with a value around 2.50% in all samples. The local WF are lactose free compared to Cerelac (3.13 mg.100g-1 lactose). Vitamins composition indicated retinol content varying from 1112.33 IU.100g-1 (VF) to 2668.23 IU.100g-1 (SP) and is 1083 IU.100g-1 for the Cerelac. The thiamin content varied from 106.90 μg.100g-1 to 160.80 μg.100g-1 respectively for DB and VF weaning flour. The same observations were made for riboflavin, 247.52 μg.100g-1 (VF) to 292.54 μg.100g-1 (SP). Amino acids profile indicated Essential amino acids were significantly higher compared to FAO/WHO Recommended Pattern for children, in valine (5.12-5.9g/100g Protein), Isoleucine (4.18-4.36 g/100g Protein) and histidine (2.04-2.68 g/100g Protein). Lysine content of local WF (4.86-5.9 g/100g Protein) was lower to cover the recommended pattern. Microbiological load for local WF and Cerelac determine the wholesomeness of WF for consumption as the Coliform counts were in acceptable range. At the end, we can say the values of minerals and vitamins content measured were well above the values recommended to cover Recommended Daily Allowance. Hence, local WF Processed and Sold in The Mount Cameroon Region are good for children’s normal nutrition and growth if handled with optimum care.
Keywords: Local weaning food, Mount Cameroon, Malnutrition, Chemical, Composition, Microbiological load
Cite this paper: Asoba Gillian Nkeudem, Sumbele Irene Ule Ngole, Anchang-Kimbi Judith, Teh Rene Ning, Samuel Metuge, Kaptso Kuaté Giscard, Nutritional Evaluation of Commonly Used Local Weaning Food Processed and Sold in the Mount Cameroon Region, International Journal of Food Science and Nutrition Engineering, Vol. 8 No. 6, 2018, pp. 131-141. doi: 10.5923/j.food.20180806.01.
Article Outline
1. Introduction
- Malnutrition has become one of the major world health problems facing developing countries. Throughout the developing world, malnutrition affects almost more than 800 million people, or 20% of the world population [1, 2]. Clinically, malnutrition is characterized by inadequate or excess intake of protein, energy, and micronutrients such as vitamins, and the frequent infections and disorders that result [3]. In developing countries, most of the complementary foods are based on local staple foods mainly produced from cereals and given in liquid gruel form for infants [3, 4]. To be suitable for the feeding of young children, these cereal-based weaning foods are prepared in liquid form by dilution with a large quantity of water, thereby resulting in more volume but with a low energy and low nutrient dense food [3]. These cereal-based gruel forms are poor in nutritional value as they lack the essential amino acids such as threonine, lysine and tryptophan [4].The weaning period is a crucial period in an infant’s life. At the age of 5–6 months, most infants begin to eat supplementary semi solid foods. At this stage homogenized infant foods play a major role in their nutrition [5]. Weaning foods (WFs) for a child in a developing country like Cameroon where WFs are relatively expensive, is out of reach of a majority of the people and may result in malnutrition thus pose a risk to the life of a child, especially if the parents are low-income earners. Weaning includes the long critical period when the child slowly gets used to other adults' food while continuing breast feeding. Hence, it is a vulnerable period of life and particularly so when food resources are limited. Death and morbidity due to Protein Energy Malnutrition (PEM) in infancy continue to scourge populations in many parts of the third world. Dietary data in many parts of these countries reveal low intake of energy at this critical time of human development. Some nutritionists have argued that Recommended Daily Allowance (RDA) for incidentally weaning infant, energy may be set too high. Intake of many infants in the third world is considered too far below the international RDA.In developing countries, these children often falter in growth and become anaemic and deficient in vitamin A since household-level weaning foods introduced do not provide adequate micronutrients, good nutrition, particularly during infancy and childhood can promote adequate physical and mental development. Certain nutrients such as protein, fats and oils in food maintain life; thus used for cell growth, repair, and regulation of function [6]. Emerging evidence indicates that diseases such as hypertension, cardiovascular diseases, respiratory diseases, and diabetes are related to poor health and nutrition of the infant; thus, the need to provide a low-cost, nutritious weaning supplement for infants cannot be overemphasized [7]. Baker, 1994 argues that malnutrition during infancy permanently changes the body’s structure, physiology, and metabolism, leading to coronary heart diseases and may lead to stroke later in life. How infants are feed appears to influence their long-term development and health [7], thus heightening the importance of improving infant food. The traditional local weaning foods could be improved upon by combining locally available foods that complement each other in such a way that new patterns of amino - acids created by this combination is similar to that recommended for infants [8-10]. Soya beans have recently become popular in the West African sub-region due to their high protein content and quality, and is being cultivated at an increasing rate. It has many advantages over animal products. Hence, there will be improvement of the nutritional value of the food as well as the nutritional status of the consumer (the infant) if both cereals and legumes are blended in the preparation of the food. Soybeans, groundnuts and maize are locally produced and this may make the soy weaning product very affordable. Generally in Cameroon and in the South West region in particular, comprehensive information and data about the quality of weaning foods processed and sold in the market and types given to infants are still lacking. Weaning patterns in the country are still found to vary widely due to regional differences in food supplies and food habits. This study was therefore carried out to assess the quality of some local weaning food formulae processed and sold in the Mount Cameroon region, from nutritional point of view.
2. Material and Methods
2.1. Sample Collection
- Samples of some local weaning food namely: Vita Force (VT), Soytine (ST), Soyaconia (SC), Soya Pap (SP), Tanty Reine (TR), Dina Baby (DB) were obtained from local markets in Muea, Ekona and Dibanda in the South West Region of Cameroon in their respective packaging form, while a standard of Cerelac® an infant cereal milk biscuit produced by Nestlé© was obtained from the pharmacy and transported to the laboratory where they were packed and sealed in polyethylene bags at ambient temperature (26 ± 2°C) and 760 mmHg until further analysis.
2.2. Nutritional Analysis
- The commercial local weaning food formulae were analyzed for chemical and microbiological properties.
2.2.1. Moisture Content
- Five grams of each sample was weighed into a previously dried and weighed glass crucible. The crucible and its content was placed in a thermostatically controlled oven at 105°C for five hours. It was cooled in a desiccator and then weighed. The procedure was continued until a constant weight was obtained. The loss in weight was recorded as moisture content and it was expressed as a percentage of the total weight of sample used [11].
2.2.2. Ash Content
- Two grams of the dried samples from the moisture determination was weighed into a previously dried and weighed porcelain dish. It was then placed in a muffle furnace (Gallenkamp, UK) heated to 600°C and kept constant at that temperature for six hours. The samples were removed and were then cooled in a desiccator and then weighed. The difference in weight of the residue and dish was recorded as the ash content and was expressed as a percentage of the total weight of the sample [11].
2.2.3. Crude Fat
- Two grams of the dried sample from the moisture determination was transferred into a paper thimble and plugged with cotton wool. It was then placed in a soxhlet extractor. Two hundred and fifty milliliters (250 mL) of petroleum ether (60-80°C) was measured into a previously dried and weighed round bottom flask. It was firmly attached to the soxhlet extractor, and extracted for sixteen hours on low heat. After the extraction, the flask was removed and the petroleum ether evaporated over steam bath. The flask was dried in an oven for an hour at 100°C with the door of the oven not latched. It was then cooled in a desiccator and weighed. The difference in weight of the flask gave the weight of the crude fat present in the sample. This was expressed as a percentage of the total weight of sample [11].
2.2.4. Crude Fibre
- The defatted sample was transferred into a 750 mL conical flask and 0.5g asbestos added. Two hundred milliliters (200 mL) of boiling 1.25% sulphuric acid was added to the flask and the flask connected to a cold finger condenser and boiled for thirty minutes. The contents of the flask was filtered and the residue washed with boiling water until the washings was no longer acidic (as tested with litmus paper). The charge and the asbestos were washed back into the flask with 200 mL of boiling 1.25% NaOH solution and again attached to the cold finger condenser for thirty minutes, after which it was filtered and washed thoroughly with boiling water. The residue was transferred and washed with 15 mL alcohol and dried for one hour at 100°C. The crucible and contents was cooled in a desiccator and then weighed. The crucible was then placed in a muffle furnace previously heated to 600°C and kept constant for thirty minutes and then removed and cooled again in a desiccator. The weight was taken and the difference in weight of the crucible and content gives the crude fibre content of the sample [11].
2.2.5. Protein
- Nitrogen content was determined using the Leco Nitrogen Analyzer (model FP 2000; St. Joseph, MI) which is a non- dispersive, infrared, microcomputer-based instrument. Sample (0.2 g) was weighed into the sample boat and the weight registered on the attached computer. The key designated as “analyze” was selected and the sample pushed into the combustion chamber. The furnace and the oxygen gas caused the sample to combust releasing nitrogen gas and an oxide of nitrogen. The nitrogen content was recorded and the protein content calculated using a conversion factor of 6.25 [12].
2.2.6. Carbohydrate
- The carbohydrate content in the foods was obtained by calculating the difference between the sum of all the other food nutrients analyzed and subtracted from 100 (the total nutrient composition). That means, Carbohydrate (g.100g-1) =100-[Protein (g)+Fat (g)+Ash (g)+Fibre (g)] [12].
2.2.7. Sugars Profile
- Samples of (10 μL) were analyzed by high-performance liquid chromatography (HPLC) using a Shimadzu Prominence system with Shimadzu refractive index detector and a 300 × 7.8-mm Rezex ROA-organic acid column (Phenomenex, Torrance, CA) with 0.004% HPLC-grade formic acid (pH 3.30 ± 0.02) (Sigma-Aldrich, St. Louis, MO) in water as the mobile phase at a flow rate of 0.59 mL·min−1 as described by Linden [13]. Peaks were identified and quantified based on retention times and peak area relative to authentic standards. Standards were prepared at 0, 1, and 2 mM of fructose, glucose, dextrose, lactose and sucrose. Standards measurements were performed every 20 runs and values were averaged at each concentration level to determine the slope of the standard curve.
2.2.8. Fatty Acids Profile
- Fatty acids were determined by gas-liquid chromatography with flame ionization detection (GLC-FID)/capillary column based on the method used by Oliveira et al., [14] with minor modifications. Fatty acid methyl esters (FAMEs) were prepared by hydrolysis with a 11 g.L-1 methanolic potassium hydroxide solution, methyl esterification with BF3/MeOH, and extraction with n-heptane. The fatty acid profile was analyzed with a Chrompack CP 9001 chromatograph (Chrompack, Middelburg, The Netherlands) equipped with a split-splitless injector, a FID, and a Chrompack CP-9050 autosampler. The temperatures of the injector and detector were 230 and 270°C, respectively. Separation was achieved on a 50 m × 0.25 mm i.d. fused silica capillary column coated with a 0.19 µm film of CP-Sil 88 (Chrompack). Helium was used as carrier gas at an internal pressure of 120 kPa. The column temperature was 160°C, for a 1 min hold, and then programmed to increase to 239°C at a rate of 4°C/min and then held for 10 min. The split ratio was 1:50, and the injected volume was 1.2 µL. The results are expressed in relative percentage of each fatty acid, calculated by internal normalization of the chromatographic peak area. Fatty acid identification was made by comparing the relative retention times of FAME peaks from samples with standards. A Supelco (Bellefonte, PA) mixture of 37 FAMEs (standard 47885-U) was used. In addition, the fatty acid were identified with individual standards also purchased from Supelco.
2.2.9. Energy Value
- Energy value was determined by calculation from fat, carbohydrate and protein contents using Atwater’s Conversion factors [15].
2.2.10. Mineral Determination
- Two grams of the dried samples were used in the determination according to the method of Benton and Vernon [16]. The sample was ignited in a muffle furnace at a temperature of 6000°C. The ash was dissolved in 10 mL of 5M HCl. Acid digestion of the ash was then carried out on a steam plate and the digested sample was carefully washed with distilled water and filtered using Whatman’s filter paper into a 50mL volumetric flask and diluted to volume. The samples and blanks were then directly analyzed for the different minerals using the Atomic Absorption Spectrophotometer (Perkin-Elmer Analyst 700 spectrophotometer (Norwalk, CT, USA).
2.2.11. Amino Acid Determination
- Amino acid composition of samples was measured on hydrolysates using an amino acid analyser (Sykam-S7130) based on high performance liquid chromatography technique. Sample hydrolysates were prepared following the method of Moore and Stein [17]. Two hundred mg of sample were placed in a hydrolysis tube. Then 5 mL 6M HCl were added to sample into the tube, tightly closed and incubated at 110°C for 24 h. After incubation, the solution was filtered and 200 mL of the filtrate was evaporated to dryness at 140°C for an hour. Each hydrolysate after dryness was diluted with one mL of 0.12 M, pH 2.2 citrate buffers, the same standard applied to amino acids. An aliquot of 150 µL of sample hydrolysate was injected in a cation separation column at 130°C. Ninhydrine solution and an eluent buffer (the buffer system contained sodium acetate (90%) and acetonitrile (10%) were delivered simultaneously into a high temperature reactor coil (16 m length) at a flow rate of 0.7 mL.min-1. The buffer/ ninhydrine mixture was heated in the reactor at 130°C for 2 min to accelerate chemical reaction of amino acids with ninhydrine. The products of the reaction mixture were detected at wavelengths of 570 nm and 440 nm on a dual channel photometer. The amino acid composition was calculated from the areas of standards obtained from the integrator and expressed as percentages of the total protein.
2.2.12. Vitamins Determination
- 1) Determination of Water-Soluble VitaminsVitamin B: The vitamin B group was extracted according to a previously described method [11]. In brief, okra powder (2 g) was placed in 25 mL of H2SO4 (0.1 N) solution and incubated for 30 min at 121°C. Ten, the contents were cooled and adjusted to pH 4.5 with 2.5 M sodium acetate, and 50 mg Takadiastase enzyme was added. The preparation was stored at 35°C overnight. The mixture was then filtered through a Whatman No. 4 filter, and the filtrate was diluted with 50 mL of pure water and filtered again through a microporefilter (0.45 µm). Twenty microliters of the filtrate was injected into the HPLC system. Quantification of vitamin B content was accomplished by comparison to vitamin B standards. Standard stock solutions for thiamine, riboflavin, niacin, pyridoxine, and cobalamin were prepared as reported previously [18]. Chromatographic separation was achieved on a reversed phase- (RP-) HPLC column (Agilent ZORBAX Eclipse Plus C18; 250 × 4.6 mm i.d., 5 µm) through the isocratic delivery mobile phase (A/B 33/67; A: MeOH, B: 0.023 M H3PO4, pH = 3.54) at a flow rate of 0.5 mL/min. Ultraviolet (UV) absorbance was recorded at 270 nm at room temperature.Ascorbic Acid: Vitamin C was extracted according to the modified method of Babarinde and Fabunmi [19]. 10 g offlour was blended and homogenized with an extracting solution containing metaphosphoric acid (0.3 M) and acetic acid (1.4 M). The mixture was placed in a conical flask and agitated at 10,000 rpm for 15 min. The mixture was then filtered through a Whatman No. 4 falter, and samples were extracted in triplicate. The ascorbic acid standard was prepared by dissolving 100 mg of l-ascorbic acid in a metaphosphoric acid (0.3 M)/acetic acid (1.4 M) solution at a final concentration of 0.1 mg/mL. The calibration line was converted to a linear range based on four measured concentration levels. Quantification of ascorbic acid content was performed on an Agilent HPLC system. Chromatographic separation was achieved on an RP-HPLC column through isocratic delivery of a mobile phase (A/B 33/67; A: 0.1 M potassium acetate, pH = 4.9, B: acetonitrile: water [50:50]) at a flow rate of 1 mL.min-1. UV absorbance was recorded at 254 nm at room temperature.2) Determination of Fat-Soluble VitaminsVitamin A D, E and K: In 10 g flour, 1 g of pyrogallic acid, 70 mL ethanol, and 30 mL (50%) KOH were added, stirred, and refluxed for 40 min using a water bath at 50 ±2°C [20, 21]. Double-distilled water was used to neutralize the extract, which was dehydrated using anhydrous sodium sulfate. Further, the extract was concentrated to approximately 5 mL by using a water bath (50±2°C), diluted to 10 mL by using methanol, filtered using a 0.45 membrane, and finally subjected to HPLC analysis. RP-HPLC analysis was performed with the Agilent 1100 series HPLC system (Agilent; USA), including a diode array detector. The column was made of stainless steel. For fat-soluble vitamins, the Agilent Eclipse XDB-C18 column was used (5 µm, 4.6×150 mm), the solvent was methanol, and UV detection was recorded at 325 nm for vitamin A, 265 nm for vitamin D3, 290 nm for vitamin E, and 244 nm for vitamin K3. Separation of all vitamins was based on isocratic elution and the solvent flow rate was maintained at 1 mL.min-1. Twenty microliters of oil was directly injected into the HPLC column. Fat soluble vitamins were identified by comparing their retention times with those of authentic standards. Standard solutions of vitamins were prepared by serial dilution to concentrations of 0.1, 1, 2, 5, and 10 mg per liter of vitamins D3, E and K3, respectively. Twenty microliters of standard solution was injected, and peak areas were determined to generate standard curves [22].
2.2.13. Microbiological Analysis
- Coliform count and total viable count / standard plate count/aerobic plate count for local weaning food.Test was done for the minus one dilution and without any dilution (direct). For the direct pouring point one milliliter from each sample was transferred into sterile petri-dish which was kept in a Heraeus T 5042 K drying oven (Tamson, Zoetermeer, Holland) for 2 h. 0.1 mL from 10-1dilution series was poured into another petri-dish for checking the coliform count for 10-1dilution of the sample. Then 12 mL of Violet Red Bile (VRB) agar 48±1°C. The agar was then allowed to solidify at room temperature (27°C). The plates were incubated in an inverted position at 37°C for 48 h in a Fisher 322 incubator (Scientifc Company, USA). The colonies were counted manually and the results were expressed as the number of Colony Forming Units (CFU) per gram.
2.2.14. Statistical Analysis
- Data obtained was subjected to statistical analysis. Means, Analysis of Variance (ANOVA) were determined using the Statgraphic Version 6.0 and the differences between the mean values were evaluated ATP < 0.05 using Duncan’s multiple range test.
3. Results and discussion
3.1. Macronutrient Composition
- Table 1 shows proximate composition of the different local formulated weaning foods for the crude protein, ash, crude fibre, fat, moisture, carbohydrates and energy. VF, ST, SC, SP, TR, DB, had significantly higher moisture contents (P<0.05) when compared to moisture content of Cerelac (3.10%). Moisture contents are within the recommended value (5-10%). The range was from 3.1g.100g-1 to 8.87 g.100g-1. The formulation TR had the highest moisture content (7.87g.100g-1) while the control Cerelac had the least (53.1g.100g-1) moisture content. Low moisture content of food samples is desirable for extending the shelf life of food products while high moisture contents in food samples encourage the growth of microorganisms; hence it leads to food spoilage. Moisture content of food is an important index of their susceptibility to microbial spoilage. When the moisture content is high, it encourages the growth of microorganisms. Moisture content would therefore indicate low growth of bacteria and fungi. [9, 10]. Moisture content is used as a quality factor for prepared cereals which should have 3-8g.100g-1 moisture content [23], therefore the maximum moisture content obtained in diet TR is 7.87g.100g-1. The relative increase in the moisture content in local weaning food may be attributed to a variation in the treatment during the drying process of the diets and the storage condition. The total ash content of the local weaning food samples varied significantly (P<0.05) higher from 2.53g.100g-1 to 3.74g.100g-1. The weaning food formulae DB and Cerelac had the least total ash content (2.53 and 1.03g.100g-1 respectively) when compared with the formulated food samples taken by VF,ST, SC, TR and SP. According to Munasinghe et al. [24] the ash content of weaning food should not exceed 5g.100g-1. However, in this experimental study, all values are acceptable but high in some case than the recommendation of FAO/OMS [25]. Protein content for both Local formulated weaning food samples and Cerelac are varied significantly (p<0.05) from 11.01g.100g-1 to 18.91 g.100g-1. The formulae DB had the least (11.01g.100g-1) protein content while SP had the highest protein content (18.91g.100g-1) and the protein content was higher in all local formulae except for DB formulae compare to the recommendation of FAO/OMS [25]. These value of protein content are in the same range of the value reported by Tiencheu et al. [10]. Proteins are important both in quantity and quality, for rapid growth and development of a child. The poor protein levels of traditional complementary foods have been a major concern in infant feeding. Use of the formulation could serve as a practical means of upgrading the protein levels of the traditional sorghum and maize based complementary foods.
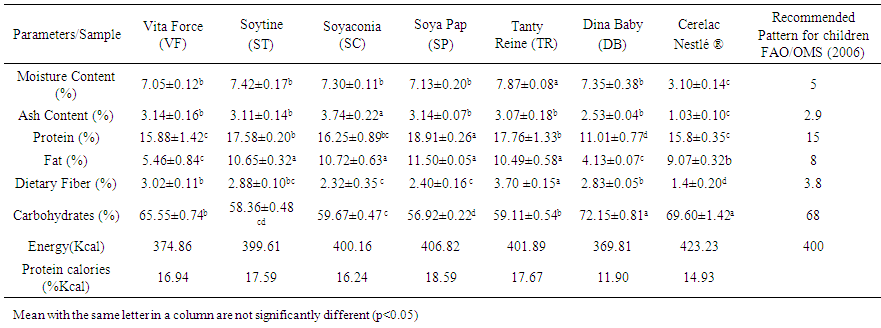 | Table 1. Proximate macronutrient content of local weaning food and Cérélac Nestlé ® (g.100g-1 DM) |
3.2. Sugar and Fatty Acid Profile of Local Formulated Weaning Food and Cerelac
- The results of sugar profile of local weaning food are presented to Table 2. According to this table, The Local weaning foods were lactose free with lactose content lower than 0.01M. This was not the case with Cerelac with a lactose level of 4.13g.100g-1. This could be explain by the fact that milk are no generally used in local weaning food formulation compare to imported weaning where milk who contain lactose. For all the others compounds, the results shown that dextrose and fructose was the main sugars. Dextrose varied from 5.7 g.100g-1 (Cerelac) to 9/74 g.100g-1 (Dextrose). The concentration of fructose varied for local weaning food from 3.88 to 5.07 g.100g-1. The lowest value was obtained with SP (3.88 g.100g-1) and the higher value by DB (5.07 g.100g-1). However the higher value was obtained with standard Cerelac who had 7.07 g.100g-1.
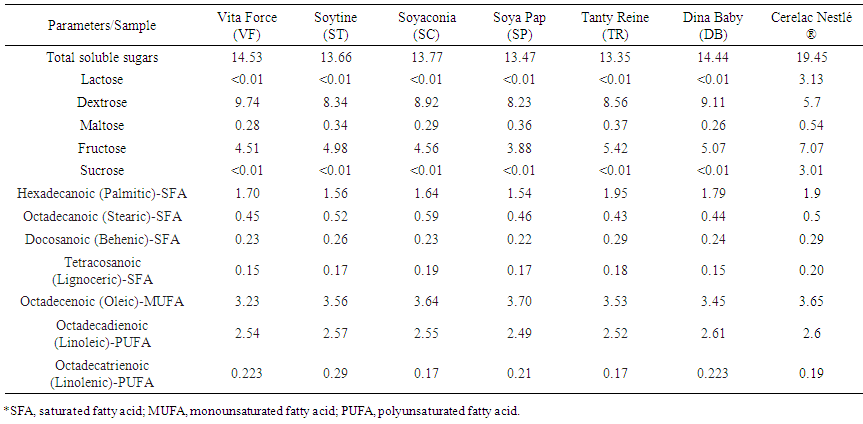 | Table 2. Sugar and Fatty acid profile of local formulated weaning food and Cérélac Nestlé ® (g.100g-1 DM)* |
3.3. Mineral Composition
- The mineral content of local weaning food are presented on Table 3. Potassium and calcium are the most abundant minerals in samples flours. Potassium and calcium contents range from 504.26mg.100g-1 (VF) to 669.34 mg.100g-1 (SP) and 230.48 mg.100g-1 (VF) to 322.30 mg.100g-1 (TR) respectively. The other minerals and their contents are: phosphorus 230.48 mg.100g-1 (VF) to 322.302 mg.100g-1 (TR), magnesium, 108.04 mg.100g mg.100g-1 (TR) to 140.43 mg.100g-1 (SP), sodium, 11.29mg.100g-1 (DB) to 2.01 mg.100g-1 (TR); zinc, 1.91mg.100g-1 (DB) mg.100g-1 to2.45 mg.100g-1 (TR); manganese, 2.97mg.100g-1 (ST) to 5.60 mg.100g-1 (TR) and iron 17.32mg.100g-1 (DB) to 21.08 mg.100g-1 (VF). Copper and iodine are the least abundant minerals in infant flours with grades ranging from 210.30µg.100g-1 (VF) to 248.62 µg.100g-1 (SP) and 18.44µg.100g-1 (DB) and 24.27 µg.100g-1) (SP). The calcium contents of the samples analyzed (325.13-400.89 mg.100g-1) are in the same range as the calcium level of the Cerelac and comparable to the calcium levels of the infant foods recommended by FAO/OMS [25]. In humans, Calcium plays a major role in the constitution of the skeleton, and also in various metabolic functions such as muscle activity, nerve stimuli, enzymatic and hormonal activities and oxygen transport [30]. The phosphorus contents of the flours analyzed (230.48-322.30 mg.100g-1) are higher than those of the maize porridge (171.32 mg.100g-1) consumed in Nigeria [31]. Phosphorus combines with calcium in the form of calcium phosphate, a hard substance that gives the body its rigidity. Phosphorus is necessary for the production and use of energy, the preservation of bones and teeth. Potassium levels (504.26-669.34 mg.100g-1) are higher than the potassium content (217.78 mg.100g-1) of maize porridge prepared in the Far North of Cameroon and Nigeria [31, 32].
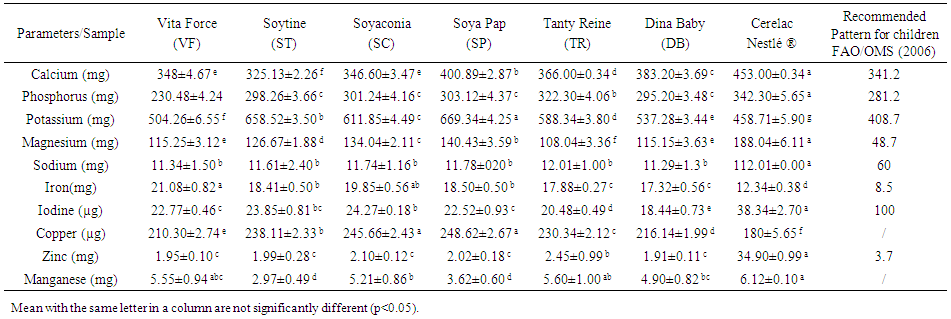 | Table 3. Mineral content of local weaning food and standard Cerelac (per 100DM) |
3.4. Vitamins Composition
- The vitamins composition of the local weaning food is presented in table 4. This composition were statistical difference for all vitamins (P < 0.05). According to this table, the retinol content varied from 1112.33 IU.100g-1 (VF) to 2668.23 IU.100g-1 (SP) and is 1083 IU.100g-1 for the Cerelac. The thiamine content varied from 106.90 μg.100g-1 to 160.80 μg.100g-1 respectively for DB and VF weaning flour. The same observations were made for riboflavin, 247.52 μg.100g-1 (VF) at 292.54 μg.100g-1 (SP). The values obtained for our different local weaning food are well above the values recommended by the FAO/OMS [25]. For niacin, pantothenic acid, pyridoxine the same observations were made with rates in these different compounds weak to cover the need for these nutrients in children. The biotin composition shown that this varied from 22.85 μg.100g-1 to 44.13 μg.100g-1 respectively for DB and TR local weaning food. This content is sufficiently high to cover the biotin requirements recommended by the FAO/OMS [25]. In the case of folic acid, vitamin C, and D, the levels obtained in the different local samples and Cerelac standards are low to cover the needs recommended by FAO/OMS [25]. All the other vitamins in the different local weaning food for cobalamin (0.54-0.78 μg.100g-1), vitamin E (1.52-2.58 μg.100g-1) and vitamin K (12.89-16.78 μg.100g-1) were higher than the values recommended by FAO/OMS, 2006. The results obtained for vitamins A, C and E agree with values obtained for weaning food processed from cooking banana, supplemented with cowpea and peanut by Bassey et al., [29]. This observation indicates that formulated food samples would serve as a good source of vitamin particularly vitamin E which is essential for cognitive development and blood coagulation and development in infant and children.
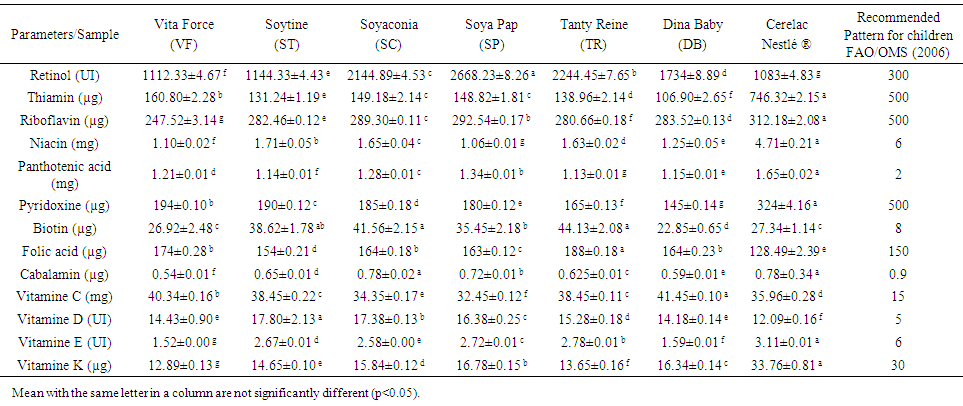 | Table 4. Vitamins content of local weaning food and standard Cerelac (per 100DM) |
3.5. Amino Acids Profile
- Amino acids content is shown in Table 5. Essential amino acids were significantly higher compared to FAO/WHO Recommended Pattern for children in Valine (5.12-5.9g/100g Protein), Isoleucine (4.18-4.36 g.100g-1 Protein) and Histidine (2.04-2.68 g.100g-1 Protein). In the case of Lysine (4.86-5.9 g.100g-1 Protein) DB (4.86 g.100g-1 Protein) content was lower to cover the recommended pattern children. The same observation was made with Threonine (3.65-4.3 g.100g-1 Protein) where ST, SC and SP with 3.78, 3.65 and 3.78 g.100g-1 Protein respectively, However the values for Methionine (2.22-2.38 g.100g-1 Protein) and Phenylalanine (4.77-5.28 g.100g-1 Protein) did not have a recommendation for FAO.OMS. The value of essential amino acids obtained were lower compared to their amounts of data reported by Larsson-et al., [35]. In the case of non-essential amino acids, Table 5 shows that Arginine varied from 3.21 to 4.10.100g-1 Protein for SP and ST respectively. Aspartic acid varied from 4.00 to 5.15 g.100g-1 Protein for DB and ST respectively. In general the same observation was made for Serine, Glutamic acid, Proline, Glycine, Alanine, Cystine and Tyrosine. The amounts of amino acids (Lysine, Methionine, Phenylalanine, Glutamic acid, Tyrosine and Arginine) in this study were higher, whereas the other aminoacids were lower compared to the results reported by [35].
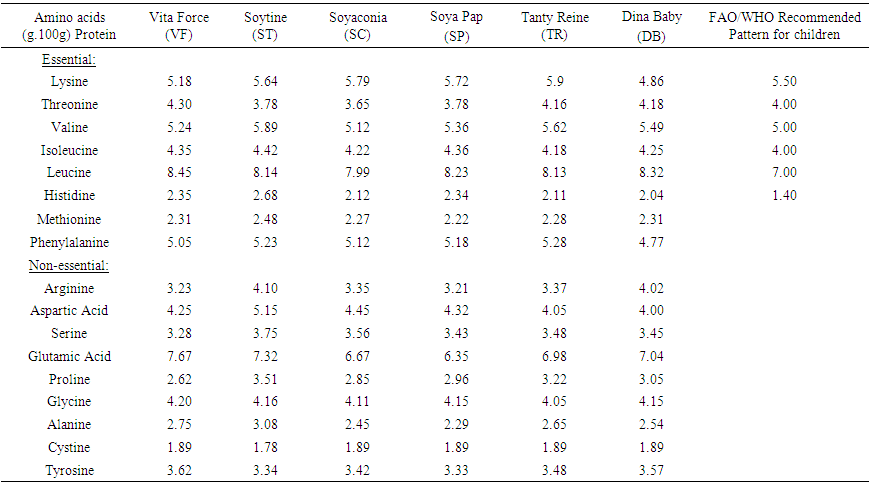 | Table 5. Amino acid profile of local weaning food compared to FAO/WHO Recommended Pattern |
3.6. Microbiological Load of for Local Weaning Food Processed Food and Cerelac
- Microbiological analysis was conducted for weaning food to determine their wholesome for consumption. In the Microbiological quality analysis, Coliform counts were in acceptable range (Table 6). According to the recommendations of UK Food Protection Agency and Food Standard Australia New Zealand (FSANZ), acceptable Total Plate Count (TPC) for cereal flour mixtures is < 107 CFU/g and coliform count is < 3 CFU/g and formulated weaning foods were in that acceptable range. The spoilage of many foods may be imminent when the total viable count reaches 10-100 million per gram of product. The international microbiological standard recommends a bacteria contaminants limit of less than 106 cfu.mL-1 for food. The high bacteria counts obtained may be due to poor personal hygiene of vendor and a lack of good manufacturing practices during the food formulation process.
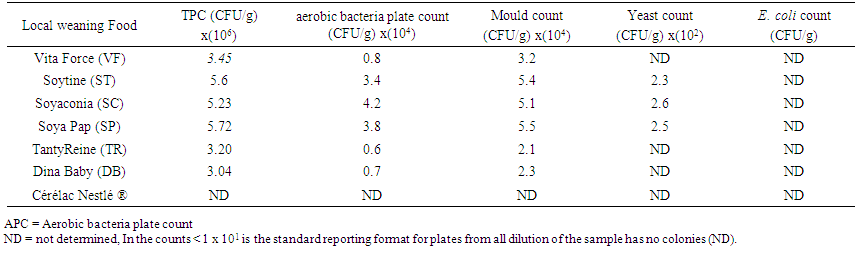 | Table 6. Microbiological load for local weaning food processed food and Cerelac* |
4. Conclusions
- After carrying out the nutritional analysis of these local weaning foods, we realized that Vita Force, Soytine, Soyaconia, Soya pap, Tanty Reine and Dina Baby had higher moisture content when compared to the standardized moisture content of Cerelac and the moisture content was within the recommended value. The protein content of weaning foods varied significantly with that of Cerelac, with DB having the least protein content. The local weaning foods were lactose free. Potassium and calcium were the most abundant minerals present in the local weaning foods. The values of vitamins content measured were well above the values recommended. The essential amino acids were significantly higher compared to FAO/WHO recommended pattern for children. Finally it was also observed during the microbiological quality analysis that, the formulated weaning foods were within the accepted range of food protection free from microorganisms. However, the bacteria counts were slightly high which may be due to poor personal hygiene. Hence we can conclude that, local weaning foods are good for children’s normal nutrition and growth if handled with optimum care.
ACKNOWLEDGEMENTS
- We gratefully acknowledge the Department of Food science, University of Pretoria for some chemical analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML