-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2018; 8(5): 127-130
doi:10.5923/j.food.20180805.03

Antibiotic Resistance in Salmonella spp. Isolated from Local Chickens in Saudi Arabia
Saeed Abdullah Badahdah1, Mosffer Mohammed Aldagal2
1Department of Food Science and Technology, Nasser's College for Agricultural, Aden University, Yemen
2Department of Food Science and Human Nutrition, Food Sciences and Agriculture, King Saud University, Kingdom of Saudi Arabia
Correspondence to: Saeed Abdullah Badahdah, Department of Food Science and Technology, Nasser's College for Agricultural, Aden University, Yemen.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Salmonellosis is considered to be one of the major causes of public health problems worldwide and it is considered to be one of a leading cause of food poisoning in humans which causes various diseases among human been. The present study was carried out to assess the prevalence of Salmonella that isolated from locally chilled chickens in Saudi Arabia. A total of 198 samples were purchased from elven different local companies named from A to K in Riyadh city. These samples divided into two groups where each group consist of 99 samples. The first group samples were taken directly without incubation in another hand, the second group samples were stored at 6°C for 4 days. The results show that the 76.76% and 67.0% positive Salmonella shows in the first and second groups respectively. Out of 198 samples processed from the both groups, 145 isolates of Salmonella were obtained, of which 55.8% belonged to S. Typhimurium and 23.4% belonged to S. Enteritidis and the remainder 20.6% considered as other species of Salmonella. Then these all positive samples for both species either S. Typhimurium and S. Enteritidis were undergo to Antibiotic resistance test. The results revealed that S. Typhimurium was highest resistant to Chloramphenicol (C30 µg) and least resistant to Streptomycin (S10 µg), on the other side S. Enteritidis was highest resistant to Neomycin (N30 µg) and least resistant to Tetracycline (TE30 µg). This study has shown that poultry chilled chickens harbour Salmonella spp. which can cause a lot of food poisoning in humans.
Keywords: Prevalence, Salmonella, Chicken, Local, Saudi Arabia, Antibiotic, Resistance
Cite this paper: Saeed Abdullah Badahdah, Mosffer Mohammed Aldagal, Antibiotic Resistance in Salmonella spp. Isolated from Local Chickens in Saudi Arabia, International Journal of Food Science and Nutrition Engineering, Vol. 8 No. 5, 2018, pp. 127-130. doi: 10.5923/j.food.20180805.03.
Article Outline
1. Introduction
- Foodborne illnesses are defined by the World Health Organization (WHO) as diseases which are toxic or infectious in nature. The latter illnesses are caused by an agent that enters the body through the ingestion of food (Velusamy et al., 2010). There is an underestimation of foodborne diseases incidence because they may not be reported and many outbreaks of food poisoning cases are misdiagnosed (Mor-Mur et al., 2009). 37.2 million episodes of foodborne illness are estimated to occur annually in the United States (Scallan et al., 2011). The majority of foodborne outbreaks are caused by Salmonella spp, Listeria monocytogenes, Escherichia coli O157:H7 and Campylobacter (Velusamy et al., 2010). Salmonella and Campylobacter are prevalent in poultry and are also considered two of the most prevalent foodborne pathogens worldwide (Heur et al., 2001). Salmonella is one of the most important bacterial pathogens responsible for food poisoning in humans. The organism has been isolated from a range of foods such as meat and dairy products in almost every country in which it has been studied. It has been estimated that 90% of cases of salmonellosis are acquired from food. Salmonella spp, are Gram-negative non-spore forming organism with two species causing illness in humans, S. Enteritidis and S. Typhimurium more frequently isolated from chicken carcasses and the most common cause of salmonellosis in humans (Carrasco et al., 2012). Antibiotics have been successfully used in humans and veterinary medicine as food animal growth promoting agents, prophylaxis and therapeutics However, their indiscriminate use has created enormous pressure for selection of antimicrobial resistance among bacterial pathogens worldwide, mainly in Salmonella strains isolated from poultry and poultry environment (Fey Pd et al., 2000). Few studies on Salmonella in the Saudi Arabian food market have been performed. Therefore, this study investigates the prevalence of Salmonella in chicken local product in Saudi Arabia than classification of salmonella isolates from chicken and study of antimicrobial resistance profiles for the isolates.
2. Materials and Methods
2.1. Samples Collection
- Whole chicken carcasses (n=99) were obtained from a wholesale poultry market located in the northern part of Riyadh City, Saudi Arabia, the samples were collected from 11 major national poultry companies (A, B, C, D, E, F, G, H, I, J, K). The samples were refrigerated and transported to Food Microbiology Laboratory, College of Food and Agricultural Sciences, King Saud University and kept refrigerated until the beginning of the experiment.
2.2. Experimental Design
- Chilled chicken samples were divided into 2 groups: The first Group A: included 99 chickens ‘samples, the rate of 9 per chicken company, were tasted for the microorganism at 1 day after purchase date. The second Group B: included 99 chickens' samples, the rate of 9 per chicken company were tasted for microorganisms after storage for 4 days at 6°C (4 days after the day of purchase).
2.3. Isolation and Identification of Salmonella spp.
- Salmonella spp. were isolated according to (ISO, 5679, 2002). By rinsed carcass into a sterile plastic bag with addition equal volume of lactose broth (CM0137, Oxoid), then incubated at 37°C for 16 to 20 hours. After incubation, 1 mL of the pre-enriched sample was transferred into 9 mL of Selenite Cysteine Broth Base (CM0699, Oxiod) and incubated at 42°C for18 to 24 h. Following Incubation, a loopful of each culture was streaked onto Xylose Desoxycholate Agar (XLD, CM046, Oxiod) and salmonella Shigella agar (SS, CM0099, Oxiod), Presumptive Salmonella colonies chosen from each plate were inoculated onto nutrient agar (CM0309, oxiod) and grown overnight at 37°C. Salmonella isolates were screened biochemically using triple sugar iron. Colonies that exhibited typical reactions were further biochemically characterized using API 20E (Biomerieux, France) as recommended by the manufacturer.
2.4. Classification of Salmonella
- Typical Salmonella isolated by microtiter agglutination test to differentiate between salmonella Typhimurium and salmonella Enteritidis. This method also called the Kaufmann and White scheme (Grimont & Weill, 2007).
2.5. Antimicrobial Susceptibility Test
- The antibiotic susceptibility of isolates was determined according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2006). Agar diffusion assays were performed on Muller–Hinton agar with disks containing 8 different antibiotic agents (Oxoid, UK). The antibiotics tested were as follows: Ampicillin (AMP 10µg), Nalidixic acid (NA 30 µg), Tetracycline (TE30 µg), Streptomycin (S10 µg), Chloramphenicol (C30 µg), Sulfamethoxazole (RL25µg), Amoxicillin (AML25 µg), Neomycin (N30 µg). The interpretive categories susceptible, intermediate or resistant were used according to CLSI guidelines (CLSI, 2010).
3. Results and Discussion
3.1. Detection of Salmonella in Chicken
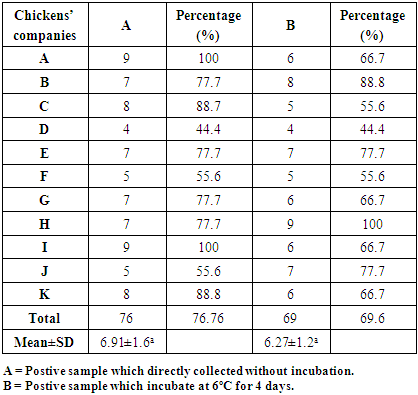
- Table (1) shows that the Percentage of salmonella spp. which isolated from 11 local chickens companies. According to the table, the total percentage of Salmonella spp. in group A were 76.76% (76 of 99 samples), that mean 24% (23 out of 99 samples) were clean, in other words, they do not infected by salmonella spp. On the other hand group B 69.6% (69 of 99 samples) were salmonella spp. isolated while 30.3% (30 out of 99 samples) were not. So, there was not significantly difference (p≥0.05) between group A and B. However this study also revealed salmonella spp. that found in these samples considered as the highest level compare with previous studies. For instance, in Saudi Arabia by (Al-Nakhli et al., 1999) the isolate salmonella spp. in poultry samples were 4% included (birds, feed, breeders of poultry and craps). Also, in Saudi Arabia by (Iyer et al., 2013) isolated salmonella spp. in samples were 45%. Moreover, there are many studies conducted in this field that showed high prevalence salmonella spp. in chicken. For example, in Tunis (Abbassi, I et al., 2012), the percentage of isolated salmonella spp. from chickens samples were (48.3%). As well as in China, they were reached to 55% in chilled chicken samples (Zhu et al., 2014). Also in Brazil, (Medeiros et al., 2011) showed 50.6% of the samples were positive for Salmonella. The high rate of Salmonella in poultry showed that it becomes a serious problem in Saudi Arabia and in many countries. On the other hand, other studies showed that low prevalence of salmonella isolates in chickens. For example, in France the percentage salmonella in chicken’s slaughterhouses were 7.52% (Hue et al., 2010), and also in South Korea, they were 3.7% (R.-H. Yoon et al., 2014). As a consequences of various studies that explained widespread of salmonella spp. in chickens’ samples, for this it will be a big issue in the future not merely in Saudi Arabia but also around the world. Therefore, it is necessary to conduct on research field in depth to identify the source of this microbe and how it is resistance to different antibiotics and to get experience from the countries that tackled with this issue in the past and also that recorded the lowest rate of chicken isolated salmonella spp.
|
3.2. Classification of Isolated Salmonella spp. from Chicken Sample
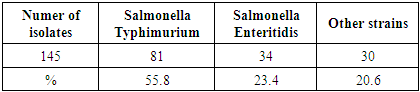
- The table (2) shows that the result of classification of salmonella spp. isolated from 145 chicken samples which divided in two groups according to the prevalence in chickens. The vast majority was salmonella Typhimurium 55.8% (81 out of 145 samples) while salmonella Enteritidis was 23.4% (34 out of 145 samples). Several studies have confirmed that Salmonella Typhimurium and salmonella Enteritidis were the most significant species that found in chickens samples rather than other species. To illustrate, in South Korea by (yoon Ran-Hee et al., 2014).
|
3.3. Antibiotic Resistance
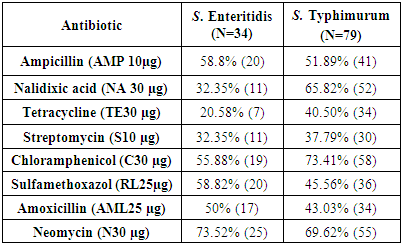
- Table (3) shows that the percentage for resistance two strains of salmonella spp. from locally chicken samples in Saudi Arabia for 8 types of antibiotics. The table shows rate of resistance bacteria S. Enteritidis for antibiotics: Ampicillin, Nalidixic acid, Amoxicillin, Streptomycin, Chloramphenicol, Neomycin, Sulfamethoxazole were (20.58, 32.35, 32.35, 50, 55.8, 58.5, 58.85, 73.52%) respectively, whereas the ratio of the least one is (20.58%) it means number of isolates resistance to antibiotics (Tetracycline) and the highest percentage (37.52%) it means a higher number of isolated resistance to antibiotics (Neomycine). Similarly, for S. Typhimurium (37.79, 40.50, 43.03, 45.56, 51.89, 65.82, 69.62, 73.41%). respectively, whereas the ratio of the least one is (37.79%) it means less number of isolates resistance to antibiotics Streptomycin, the highest percentage means the higher number of isolates resistance for antibiotics Chloramphenicol. As compared to similar studies where isolated S. Enteritidis and S. Typhimurium were the highest resistance to antibiotics (Ampicillin 10µg) (100%) (Soomro et al., 2011), while in the current study of resistance were (51.89, 58.8%) respectively. In the antibiotic (Tetracycline 30µg) the proration of resistance were (25 and 93.75%) for each S. Enteritidis and S. Typhimurium respectively, (Neomycin 30µg) and (Sulfamethoxazole 25 µg) the strains showed resistance (50 and 25%) respectively. The proration of resistance strains is (25 and 6.25%) respectively, while in antibiotic (Chloramphenicol 30µg) strains showed no resistance. Parveen et al. (2007) examined the effect of a group of antibiotics on several isolated strains of chickens, including S. typhimurium showed resistance to antibiotics, including Tetracycline (86.2%), Ampicillin (62.8%), Amoxicillin, Streptomycin (55.3%) and Sulfisoxazole (3.9%), While the resistance to the antibiotics didn't show on Nalidixic acid and Sulfamethoxazole. In addition (Yang et al., 2011) about allergy of salmonella strains which isolated from chicken on antibiotics. The strains have shown high resistance for the following antibiotics: Sulfamethoxazole (67%), Tetracycline (56%) and a lower rate to antibiotics Nalidixic acid (16%), Ceftriaxone (21%), Ciprofloxacin (35%). The results shows that the between resistors salmonella isolated from poultry to antibiotics, this difference due to excessive of using of antibiotics, which produce a new salmonella strains that characterised by high resistance of most antibiotics.
|
4. Conclusions
- This study was conducted to estimate the apparent prevalence of Salmonella spp. in locally chilled chickens in Saudi Arabia. The study has revealed that the isolation of Salmonella spp. a relatively high ratio of 76% compared to the previous local studies. The most important strains in Salmonella spp. isolated from chicken samples salmonella Eteritidies and salmonella Typhimurium. The S.Typhimurium strain was more resistant to the antibiotic (Neomycin) and less resistant to the antibiotic, (Tetracycline) while S. Enteritidies were more Streptomycin and less resistant antibiotic (Chlormphenicol).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML