-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2018; 8(5): 107-118
doi:10.5923/j.food.20180805.01

Cultivation of Arthrospira Strains in Tropical Conditions, with Particular Reference to Ethiopia
Tiruha Karssa1, Alessio Papini2, Nor Azman Kasan3
1Department of Biology, Hawassa University, Hawassa, Ethiopia
2Dipartimento di Biologia, Università degli Studi di Firenze, Florence, Italy
3Institute of Tropical Aquaculture, Universiti Malaysia Terengganu, Terengganu, Malaysia
Correspondence to: Tiruha Karssa, Department of Biology, Hawassa University, Hawassa, Ethiopia.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Tropical soda lakes generally harbor abundance of Arthrospira sp. (Spirulina) and related phytoplankton communitiesdue to their optimal water chemistry and ecological conditions. Arthrospira fusiformis was described as the dominant species in Lake Chitu. The main aim of this study was to cultivate Arthrospira sp. isolates from Lake Chitu, Lake Abijata and Lake Shalla. Morphological features were determined using light Microscope. We found three different morphotypes (H-type, S-type and C-type) of Arthrospira sp. of Lake Chitu on the basis of selected morphological parameters. In this study it has been also confirmed that a concentration of ammonium nitrate of 0.03 M is optimal for cultivation of Spirulina. Revision of taxonomic positions of Arthrospira of Lake Chitu by combining morphological, ultrastructural and molecular methods is required using a larger number of strains isolated from different sampling points in the lake and increasing sampling from other lakes from the Rift Valley. In conclusion, Lake Chitu is a potentially interesting area for isolating Arthrospira sp.in the wild and hence to provide genetic variation of possible interest for future small to large scale commercial production or research activities.
Keywords: Arthrospira, Spirulina, Lake Chitu, Morphotypes, Soda Lakes
Cite this paper: Tiruha Karssa, Alessio Papini, Nor Azman Kasan, Cultivation of Arthrospira Strains in Tropical Conditions, with Particular Reference to Ethiopia, International Journal of Food Science and Nutrition Engineering, Vol. 8 No. 5, 2018, pp. 107-118. doi: 10.5923/j.food.20180805.01.
Article Outline
1. Introduction
- Cyanobacteria species such as Arthrospira fusiforms (A. platensis), Oocystis and Anabaenopsis were reported as dominant species in the considered sampling sites of Lake Chitu, Abijata and Shalla during 1960 to 1988 periods (Fetahi, 2016). Since then, a change in dominance of species had occurred due to environmental changes (Fetahi, 2016; Otago et al. 2016). However, Lake Chitu is known for its almost monoalgal composition of A. fusiformis (Otago and Kifle, 2014).
1.1. History and Taxonomy of Spirulina (and related names)
1.1.1. The Name “Spirulina” Used in this Article Refers to a Commercial Name
- Currently the commercially used Spirulina can be assigned taxonomically to two different names, not belonging to genus Spirulina Turpin ex Gomont, but instead to genus Arthrospira Sitzenberger ex Gomont, 1892 (Gomont 1892), that is Arthrospira platensis Gomont and Arthrospira maxima Setchell and Gardner. These two names are assigned to biological entities easily distinguishable on the basis of dimension, since A. maxima spires are much larger than those of A. platensis. Arthrospira fusiformis (Voronikhin) Komárek and Lund is a specific name assigned to samples of Arthrospira with compressed spires (it has a shape of a coiled spring), typically from some African soda lakes, such as Chitu lake.What is then to be assigned to the name “Spirulina”? We must consider that species delimitation in cyanobacteria is a complex task, also on a logical basis, in relation to the difficult application of biological species concept in prokaryotes (Dvorak et al. 2015). Nevertheless, it was possible to distinguish at least the main groups of biological entities in coiled cyanobacteria. Genus Spirulina Turpin ex Gomont was based on a typical species (Spirulina major Kützing ex Gomont) that revealed not to belong to the same genus as the Arthrospira species used in cultivation, but even to a different order (Komarek et al. 2014). As a result of this taxonomic treatment, Spirulina in the sense of the cultivated one is a commercial/vernacular name, not a taxonomically correct genus name and comprises rather strains belonging to genus Arthrospira (such as A. platensis). For this reason in our text spirulina will not be written in italics, since we refer to the commercial Spirulina, that is biological entities belonging to genus Arthrospira.Cyanobacteria are considered relatively primitive organism and are believed to have evolved about 3.5 billion years ago (FAO, 2008; Ali and Saleh, 2012). Spirulina has been harvested as a food source by Aztecs from the great soda lakes of Mexico from historical records of the 16th century (Ciferri, 1893). The Aztecs (Mexicans) call it “tecuitlatl”, meaning stone's excrement and they make it into small cakes and sold it at the local markets to be used as an addition to sauces and mixed with grains (Genene Tefera et al. 2016). Back in the early 9th century, Spirulina use had a long history in Chad during Kanem Empire (Ali and Saleh, 2012). The Kanembu population living around Lake Chad were also reported to consume a traditionally collected Spirulina cake called “dihé” which is mixed with different sauces and consumed by the majority of the Kanembu population still today (FAO, 2008). The Spirulina has been harvested traditionally from the lake by skimming the surface of the water with finely woven nets. The slurry water substance containing Spirulina is allowed to drain via a pre-prepared round hole in sandy areas leaving a round cake-like substance called “dihé”, which can be sold in local markets to be used as a source of protein by the consumers. Spirulina was established as “wonderful future food source” in 1967 for its exceptionally high quality protein (70% dry weight) content (Mogale, 2016; Bleakley and Hayes, 2017). With the start of commercialization, the first large scale production plant was established during early 1970s by Sosa Texcoco company (Belay, 2013). Despite being a bacterium (Cyanobacteria) Spirulina is usually considered as microalgae, due to its ability to photosynthesize and similar appearance. Cyanobacteria (blue-green algae) have, at first glance, a similar morphology and property with respect to microalgae. However, microalgae such as Spirulina and cyanobacteria in general have unique features, such as their principal photosynthetic pigment (chlorophyll a), oxygen as a photosynthetic product and their size (5 to 10 times higher) that distinguish them from most bacteria (Moreira, 2009; Ali and Saleh, 2012).
1.2. Morphology
- Spirulina are oxygenic, multicellular, gram negative, photolithoautotroph, filamentous and non-heterocystous cyanobacterium characterized by coiled cylindrical filaments or trichomes (Fig. 1), with a length of 200-500 μm, width between 3 and 12 μm and diameter varies from 30 to 70 μm (Ciferri, 1983). Spirulina species are reported to have three different forms as spiral, straight and wavy (Moreira, 2009). Moreover, they also have other numerous relevant cell components and inclusions such as stratified cell wall, DNA region, thylakoids, ribosomes, gas vacuoles, carboxysomes, phosphate granules and cylindrical bodies (Rangsayatorn et al. 2002; Belay, 2013; Noyma et al. 2015; Deschoenmaeker et al. 2016). However, morphology of Spirulina species is influenced by certain environmental variables such as temperature, pH, salt, light and nutrient availability (Kebede, 1997; Wu et al. 2005; Rosario and Josephine, 2015).
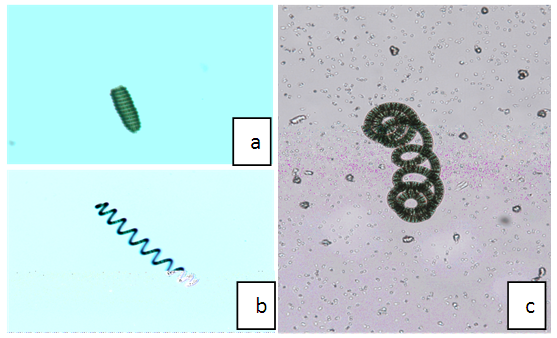 | Figure 1. Morphotypes of Arthrospira fusiformis isolated from Lake Chitu: a = H-type, b = S-type, c = C-type. Microscopic observation from the present study |
1.3. Ecology
- Spirulina species are mainly found in tropical and subtropical regions of the world water bodies having high pH (8-11), carbonate and bicarbonate (Small, 2012; Belay, 2013; Kaggwa et al. 2013). They are alkalophilic, halophilic, thermophilic and extremophilic (Mandal and Rath, 2015). The extreme conditions of water bodies enable Spirulina growth with less possibility of contamination by other microorganisms and contribute for maintenance of monoalgal cultures in large scale production using outdoor ponds (Mühling, 2000). Both natural and human induced environmental changes are reported to influence the distribution of Spirulina and may favor other species of microorganisms (Belachew et al. 2012). In addition, nutrients in natural lakes, that either come from external sources as influxes, or from inside the water bodies via upwelling, are usually limited and influence the density of Spirulina population (FAO, 2008).
1.4. Spirulina Cultivation
1.4.1. Requirements of Spirulina Growth
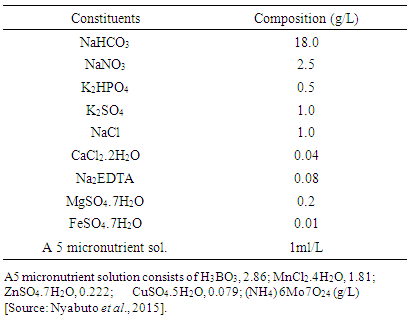
- Generally, microalgae require carbon, nitrogen and phosphorus as their major nutrients in addition to water and light for optimum growth (Harwati, 2013). Micronutrients such as potassium, magnesium, sulfur, calcium and iron are also commonly required by microalgae (Moreira, 2009; Markou et al. 2014). Zarrouk’s medium is currently in use as a standard medium for the cultivation of Spirulina and contain various constituents (Tab. 1).
|
1.5. Applications of Spirulina
- Nowadays the cultivation of Spirulina is recognized worldwide as important and profitable business in biotechnological industries and is considered a so-called super food, due to the high diversity of nutritional compositions. In addition to its use as a nutrient source for both human food and animal feed, it can also be applicable to food colorant industry, cosmetics, medicine, energy production, wastewater remediation and CO2 mitigation as explained as follows.
1.5.1. Human Food and Animal Feed
- Spirulina is becoming a known food for human worldwide being a rich source of many nutritional components such as proteins, vitamins, amino acids, fatty acids, minerals, pigments etc. (Ali and Saleh, 2012; Chu, 2012). In addition, nutritional components of Spirulina have been proven to play great role in promoting healthy body functions of consumers and thus reduce the risk of disease by enhancing the immune system (Hoseini et al., 2013; Rosario and Josephine, 2015). Its high organoleptic properties and non-toxicity make it a safe food or food supplement for human consumption. Studies have also shown that in many African countries Spirulina is directly collected from natural water bodies, dried and eaten as a cake, particularly by those who live around Lakes (FAO, 2008). Spirulina has also been proved to be a potential feed resource to many agriculturally important animal species such as chickens, pigs, cattles, ruminants, sheep and rabbits (Holman and Malau-Aduli, 2013). This last report also showed that it improves the animals’ health, growth, productivity and quality. About 50% of the current world production of Spirulina is used as feed supplement to poultry and different animals (Zahroojian et al., 2013).
1.5.2. Aquaculture
- Spirulina is also widely used in aquaculture activities as a supplement of diet mainly for fish larvae and juveniles of both zooplankton and fish (FAO, 2008; Sirakov et al., 2015). It has been also used for stabilization of the culture medium, stimulation of immune system and for their probiotic effects (Irianto and Austin, 2002). Spirulina can also be used as a source of natural pigments for the culture of prawns, salmonid fish and ornamental fish (Priyadarshani and Rath, 2012; Teimouri et al., 2013). It can also be consumed as live feeds by bivalve molluscs (e.g. oysters, scallops, clams and mussels) during all stages of their growth, whereas abalone, crustaceans and some fish species consume it during their juvenile stages (Sirakov et al., 2015).
1.5.3. Spirulina in Cosmetics Industry
- Microalgal products can also play a great role in cosmetics industry as thickening agents, water-binding agents and antioxidants (Priyadarshani and Rath, 2012; Wang et al., 2015). The extracts can also be found in face, skin and hair care products and sun protection creams (Stolz and Obermayer, 2005). Representative species commonly used in the cosmetics industry are: Spirulina, Chlorella, Chondrus crispus, Mastocarpus stellatus, Ascophyllum nodosum, Alaria esculenta, Spirulina platensis, Nannochloropsis oculata, Chlorella vulgaris and Dunaliella salina (Priyadarshani and Rath, 2012). In addition, Spirulina extracts can also be used to produce cream for the treatment of wounds in animals.
1.5.4. Microalgae and Food Colorant
- Microalgae produce various substances containing pigments such as beta carotene, astaxanthin, lutein, canthaxanthin, zeaxanthin, lycopene, bixin and chlorophyll used for coloring of food (Prasanna et al., 2007). Spirulina is a known source of natural phycocyanin which has commercial values in food industries as it is used as a natural food colorant and additive (Gouveia et al., 2008b). It can also be used as cosmetic coloring (blue color extract). However, the potential use of microalgal products as natural food coloring has limitations as it is not photo stable (Priyadarshani and Rath, 2012). In addition to being used as coloring agent in food industries, it can also give a pink color to the feather of flamingo birds which feed on Spirulina (Small, 2012).
1.5.5. Environment and Agriculture
- Microalgae have been used in bioremediation techniques to protect the environment from contaminants, hazardous substances and organic pollutants. Spirulina has been confirmed to have great role in wastewater treatment process as heavy metal remover for treating water contaminated with metals such as copper and cadmium (Small, 2012). Spirulina can also survive in highly alkaline and other extreme environmental conditions where contaminants do not grow, indicating its importance from environmental application point of view. Moreover, Spirulina can also be used as a source of biofertilizer, as it has high nitrogen and phosphorous content and thus it is important for the growth and development of agricultural plants and for soil conditioning (FAO, 2008). Therefore, Spirulina could be a cost effective alternative to chemical fertilizer in agricultural sectors (Wuang et al., 2016).
1.5.6. Biofuels
- Microalgae are considered good sources of biofuel production in comparison to other crops. Their high protein content and the rapid biomass production make them to be preferred and become an interest of researchers, entrepreneurs and the general public (Priyadarshani and Rath, 2012). The extraction of oil from microalgae (different from Spirulina) is estimated to be greater than 80% (on dry weight basis) and average annual biodiesel yield of 98.4 m3 per hectare (Rajvanshi and Sharma, 2012). Biofuel production from microalgae genera including Spirulina has been reported to reduce CO, hydrocarbons and other particulate matter emissions (Sarpal et al., 2016). Biofuel production from cyanobacteria is also profitable from the economic point of view as it can grow easily using wastes or seawater (Small, 2012).
1.5.7. Microalgae in Pharmaceutical Industries
- Products of microalgae play great role in the pharmaceutical industry. These products are mostly bioactive compounds containing carbohydrates, organic acids, amino acids and peptides, vitamins, growth substances, antibiotics, enzymes and toxic compounds of pharmaceutical importance (Rania and Hala, 2008; Chu, 2012). Spirulina produces various bioactive metabolites (primary and secondary metabolites) having antifungal, antibacterial, antiviral, antibiotic, anticancerous, antidiabetic, antianaemic and antileucopenic effects (Ali and Saleh, 2012; Vijayakumar and Menakha, 2015; Mazard et al., 2016; Sowjanya and Manjula, 2016). The co-occurrence of different algal species in the natural aquatic habitat contributes the existence of these bioactive compounds because of antagonistic interactions among them (Priyadarshani and Rath, 2012).Various studies have been done on ecology, morphology, diversity, cultivation, biological composition and applications of Spirulina by various researchers (Chu, 2012; Priyadarshani and Rath, 2012; Harwati, 2013; Marrez et al., 2014; Otago and Kifle, 2014; Hafidh et al., 2015; Nyabuto et al., 2015). But investigation on strains of potential interest for growth specifically in tropical regions is not well documented and needs to be explored. In the present study, laboratory cultivation, characterization, identification and maintenance of those strains sampled from tropical alkaline lake of Ethiopia was carried out. Moreover, malnutrition is a major problem in developing countries having high population growth resulting in high food demand. Thus, there is inadequate supply of nutrient rich foods and high children mortality rate has been reported frequently (FAO, 2008; Moreira, 2009; Albert et al., 2012). Therefore, using Spirulina as a source of those nutrients could be the best alternative to combat malnutrition in different parts of the world. In addition, Spirulina isolated from tropical climatic conditions have been reported as potential candidates as a source dietary supplements and several bioactive compounds (Ciferri, 1983; Pal et al., 2011; Harwati, 2013; Otago et al., 2014). Thus, isolation, cultivation and maintaining those potential strains is required for their potential applications such as for human and animal nutrition, energy, environment, medical and cosmetics. Ethiopian alkaline soda lakes could be a potential source of Spirulina (Fetahi, 2016; Genene Tefera et al., 2016; Otago et al., 2016). Nowadays, Spirulina has got worldwide attention for being rich source of different nutrients and their benefits. Therefore, this study would give valuable baseline information about tropical microalgae, particularly Spirulina, for further study and use.
2. Materials and Methods
2.1. Descrition of the Study Area
- Lake Abijata, Shalla and Chitu are among the tropical creater soda lakes of Ethiopia. They are located in the Ethiopian rift valley at a distance of about 285 km south of Addis Ababa at a geographical position of 7° 37′ 0″ N, 38° 36′ 0″ E, 7° 29′ 0″ N, 38° 32′ 0″ E and 7°24'13"N 38°25'16"E respectively (Legesse et al. 2002; Otago and Kifle, 2014). Lake Abijata is relatively the shallowest (<7m deep) with area of 180 km2 (Kumssa and Bekele, 2014). It is small alkaline closed lake in central Ethiopia, along the Ziway Shalla basin (Fig. 2). It lies in a saucer-shaped hollow within a deep faulted trough at an altitude of 1580 m above mean sea level (Tenalem, 2002). The water in Lake Abijata is saline and soda-type having pH of about 10 and salinity of 16.2 g/l (Tenalem, 2002; Lemma, 2016). Lake Chitu is the smallest soda lake having an area of 0.8 km2 and maximum depth of 21 m and is known for almost its monospecific population of Arthrospira fusiformis (Otago and Kifle, 2014). These authors also reported that the lake is characterized by environmental conditions such as high pH, salinity, alkalinity, Na+ and Cl- ions and limiting levels of inorganic nitrogen compounds which are proved to be ideal for the optimum growth of Spirulina species. In addition, the location of the lake is characterized by semi-arid to sub-humid type of climate with mean annual precipitation and temperature of 600 mm and 25°C, respectively (Legesse et al. 2002). Lake Shalla is relatively the deepest (266 m deep) and covering an area of 370 km2 having high saline-alkaline water but less saline-alkaline than Lake Chitu (Tenalem, 2002; Otago et al. 2014). They also reported that Lake Shalla lies at an altitude of 1550 m a.m.s.l, having a water surface temperature in the range between 22 and 26°C and less productive, although its saline-alkaline conditions are suitable for the growth of Spirulina.
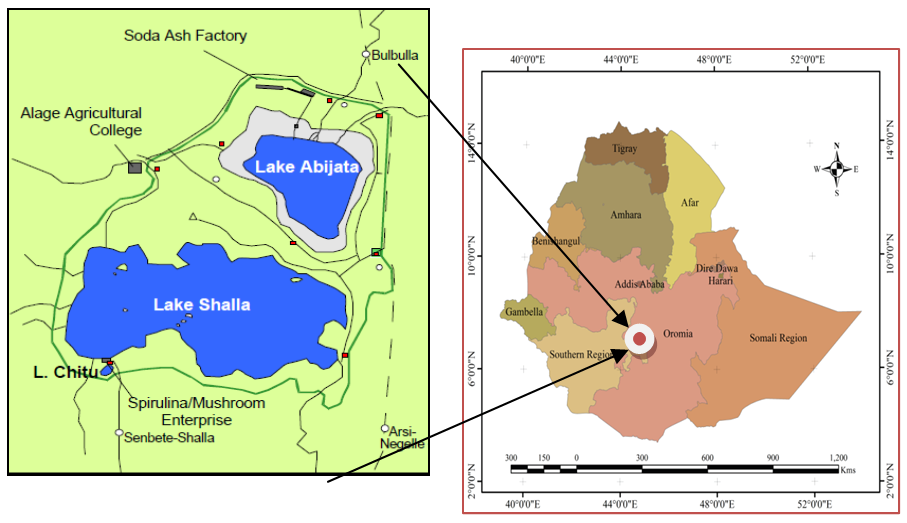 | Figure 2. Location of study sites in Ethiopia (Source: Adapted from Reaugh-Flower, 2011) |
2.2. Sample Collection
- Spirulina dominated water samples were collected 10 cm below the surface from Lakes: Abijata, Shalla and Chitu following standard methods described by (Rout et al., 2013) using plankton net and sample bottles. Salinity, pH and temperature were measured in situ using a portable water quality checker (U-10, USA). Photosynthetically Active Radiation (PAR) on the surface of the lake was also measured using photometer (HS1010, USA). After that all samples were transported to the laboratory of general botany, University of Florence, Italy for Spirulina isolation, cultivation and characterization.
2.3. Isolation and Culturing
- A few drops of samples were put on to glass slides and observed with the optical microscope to verify the presence of Spirulina, after thoroughly mixing them. Slides having Spirulina were washed with Zarrouk medium and the mixture was collected in small sterilized beaker. Afterwards, the aliquot was transferred into sterilized flasks for growth. In addition, 1-2 ml from the original sample was also taken using pipette and inoculated in to 250 ml of Erlenmeyer flasks containing sterilized zarrouk medium. All inoculated tubes were incubated in a growth chamber at 27 ± 3 °C under continuous illumination with fluorescent white lamp with light intensity of 215 μ mol photon m-2 s-2 for 30 days to allow them to grow with greater variability of morphotypes. To improve CO2 availability, agitation of the flasks and tubes containing the growth were carried out 3 times a day as proposed by Nyabuto et al. (2015). Continuous follow up of the growth conditions of cultures were done by observing their thallus under the microscope. During the process of their growth fresh sterilized Zarrouk media was also added to the cultures to improve nutrient availability for their growth. Several successive transfers have been also carried out using Zarrouk media to purify the cultures and refresh them to increase their concentration for further analysis and treatment.
2.4. Cultivation under Different Concentrations of Nitrogen Source
- The experimental organisms, Spirulina species isolated from water samples of Lake Chitu were cultivated under different concentrations of NH4NO3 using Zarrouk media. These different concentrations of nitrogen source were considered to evaluate their effect on the growth and biomass of Spirulina species according to methods described by Costa et al. (2004); Madkour et al. (2012); Sharma et al. (2014); Castro et al. (2015). The nitrogen source in the Zarrouk media sodium nitrate (NaNO3) was replaced by ammonium nitrate (NH4NO3) with concentrations: 0.01 M, 0.03 M and 0.05 M. Spirulina (10%) was added to 250 ml sterilized Erlenmeyer flasks containing sterilized Zarrouk media with (0.01 M, 0.03 M and 0.05 M) concentrations of NH4NO3. The experiment was carried out in triplicates allowing the cultures to grow under growth chamber at 27 ± 3°C for 33 days. Cultures were shaked manually three times a day with 12h: 12h photoperiod cycle and illuminance of 215 μ mol photon m-2 s-2.
2.5. Characterization and Identification of Isolates
- Characterization of the isolates based on their morphological parameters such as degree of coiling, number of coils per trichome, color of trichome, trichome end attenuation, trichome length, trichome diameter, helix diameter (at the middle and at the end) and helix pitch were carried out using optical microscope (Leica Microscopie & Systeme GmbH. Wetzlar, Germany). On the basis of their level of coiling, isolates were grouped into three different morphotypes as H-type (tightly coiled), S-type (loosely coiled) and C-type (intermediate). For each morphotype both qualitative and quantitative description of the above morphological parameters were made using optical microscope (Leica Mikroskopie & Systeme GmbH Wetzlar, Germany) at 45X and 100X magnifications. Molecular characterization using 16S rRNA sequences of the cultivated isolates was also carried out for further checking the variability among the species as described below based on standard protocols.
2.5.1. Morphological Identification Using Optical Microscope
- Drops from each triplicate samples were taken and put to glass slides. The different forms of morphotypes were identified and images were captured with a Nikon camera inbuilt in the microscope (DIGITAL SIGHTDS-LT, JAPAN). The above mentioned morphological parameters were carefully observed, measured and counted under 45X and 100X magnification power of optical microscope (Leica Microscopie & Systeme GmbH. Wetzlar, Germany). All examined morphological parameters were compared with previously described standard keys.
2.6. Growth Measurements
- Biomass concentrations of the growing cultures during cultivation were determined by measuring optical density (OD) using direct reading spectrophotometer (HACH DR/2000, U.S.A). Absorbance of Spirulina samples were read at 680 nm in the interval of three days. Each measurement of optical density was recorded to make a curve indicating Spirulina biomass concentration versus the growth period and concentration of nutrients. Optical density measurement was continued until decline phase of the cultures was attained (33 days). Protocols described by Costa et al. (2004); Harwati, (2013) and Joshi et al. (2014) were used.
2.7. Maintenance of Selected Strains
- The pure isolates of Spirulina strains were maintained using Zarrouk medium as modified (composition: 10 g NaHCO3, 0.5 g K2HPO4, 2.5 g NaNO3, 1.0g K2SO4, 1.0 g NaCl, 0.20 g MgSO4·7H2O, 0.01 g FeSO4·7H2O, 0.08 g EDTA and 0.04 g CaCl2) by (Ravelonandro et al., 2008) in Erlenmeyer flasks (250 ml capacity). During the process of growth they have been provided with a continuous illumination of 215 μ mol photon m-2 s-2 at 27 ± 3°C with manual shaking three times a day under 12:12 hour light-dark cycles (Joshi et al., 2014). The pH of the media was maintained to the optimum (9.5-10) range and checked continuously for being within the specified range. They can be maintained (preserved) for short, medium and long term (Rout et al., 2013).
2.8. Statistical Analysis
- Both SPSS (version 24) and PAST (version 3.15) statistical programs were used to analyze the morphological data obtained from this study. Morphological parameters used to characterize morphotyes of Spirulina were subjected to One-way ANOVA and Tukey’s HSD (Honest Significant Difference) test using SPSS at 95% confidence interval with marginal error of 5% to verify the variability of these parameters among and within morphotypes of Spirulina. Histograms, graphs and tables were used to display some of the main outputs of SPSS. The data obtained from optical density measurements were also subjected to One-way ANOVA using SPSS at 95% confidence interval to see statistical significant differences among growth of Spirulina under different concentrations of NH4NO3. Statistical values of P < 0.05 were considered significant.
3. Results
3.1. General Overview of Morphology and Morphological Parameters
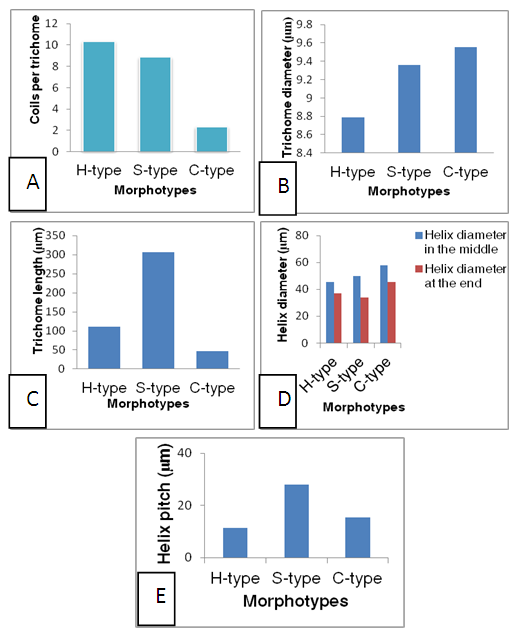
- Based on the observations with optical microscope Spirulina isolates in the present study were categorized in three morphotypes mainly on the basis of degree of coiling of trichomes and trichome end attenuation. Moreover, other parameters such as trichome length, trichome diameter, helix pitch, helix diameter and number of coils (Fig. 3) were measured and quantitatively determined. The three identified morphotypes were H-type, S-type and C-type (Fig. 4 A-C). These morphotypes may represent or share morphological characteristics between more Arthrospira species such as Arthrospira fusiformis, Arthrospira maxima and Arthrospira platensis even though they are not stable, since transformation of one morphotype to another may occur as a function of growth conditions during cultivation.
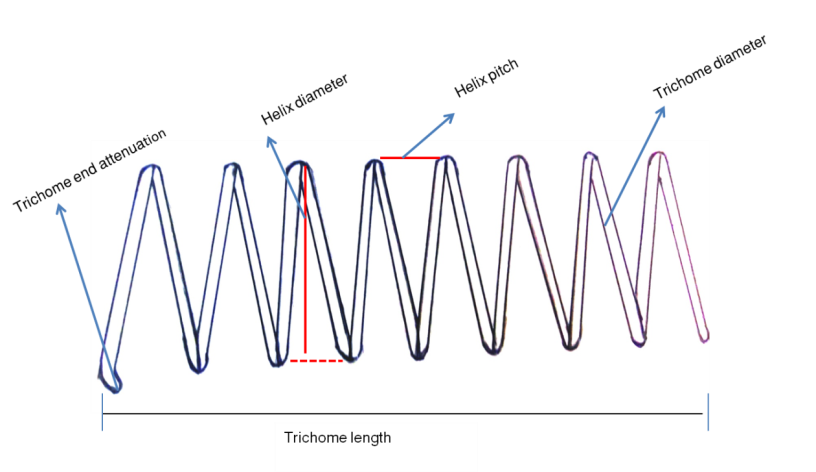 | Figure 3. Parts of Spirulina filament (morphological parameters considered in this study) |
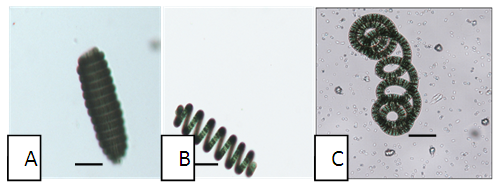 | Figure 4. Microscopic view of Spirulina morphotypes of Lake Chitu: (A) H-type; (B) S-type; (C) C-type. Scale bars = 250 μm |
3.2. Growth under Different Concentrations of Ammonium Nitrate
- We prefer to use ammonium nitrate as an alternative nitrogen source due to its cost effectivness and ease of availability in Africa. It can be locally available being used as a normal agriculture fertilizer. Therfore, it can be used for the biomass production of Spirulina in small and large scale commercial production industries. Moreover, a comparable yield and biomass production using standard medium and cheaper medium containing alternative nitrogen sources have been reported (Costa et al. 2001; Nor et al. 2015; Mashor et al. 2016). The results about the relationship between the OD and growth period graphs (Fig. 6) revealed that, the optimum growth of Spirulina depends on the concentration of NH4NO3. At the beginning of the growth period there was lower growth but it gradually increased through time. The best growth of Spirulina has been observed at 0.03 M NH4NO3 concentration in comparison with 0.01 M and 0.05 M (Fig. 6). However, the growth recorded at 0.05 M NH4NO3 concentration was significantly lower (M = 0.28 nm; P = 0.033; SD = 0.18) than values observed at 0.01 M and 0.03 M concentrations. There was no significant difference between growth of Spirulina observed at 0.01 M (M = 0.36 nm; P = 0.056; SD = 0.23) and 0.03 M (M = 0.47nm; P = 0.062; SD = 0.24) concentrations of NH4NO3. The growth in the control with NaNO3 increased continuously until day 18 unlike growth of different concentrations of NH4NO3 in which increments lasted longer (Fig. 6). Lower growth rates have been observed in the end of growth periods in 0.01 M and 0.03 M concentrations of NH4NO3 and in the control. On the contrary gradual increments towards the end have been observed at 0.05 M concentration.
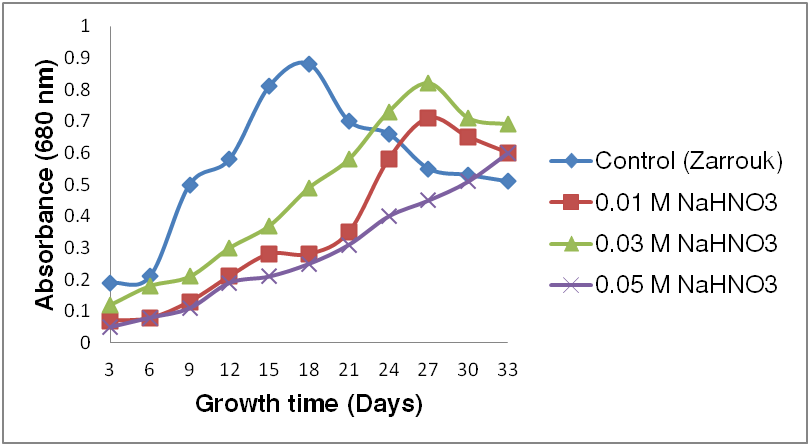 | Figure 6. Optimization of Spirulina growth using different concentrations of ammonium nitrate as an alternative nitrogen source |
4. Discussion
- Morphotypes of Arthrospira based on morphological parameters and optimization of growth conditions under different concentrations of ammonium nitrate were investigated in this study. Furthermore, morphological parameters that mainly describe the morphotypes of Arthrospira were identified and suggested to use in further studies.
4.1. General Overview of Morphology and Morphological Parameters
- Based on microscopic observation of Arthrospira isolates from the current study, three distinct morphotypes of Arthrospira as H- type (tightly coiled), S- type (loosely coiled) and C- type (intermediately coiled) have been recognized. Degree of coiling of the trichomes and trichome end attenuation were frequently used to group the morphotypes during microscopic observations. This is due to the phenotypic variation among morphotypes in terms of these two parameters. However, morphological parameters only were proved to be not reliable criteria for the taxonomic classification of Arthrospira (Jeeji Bai and Seshadri, 1980, Li et al. 2001) and see the taxonomic note in this thesis. Furthermore, most of these morphological parameters can vary with the influence of environmental parameters and nutrient conditions (Mü-hling, 2000; Kim et al. 2007; Kaggwa et al. 2013; Otago and Kifle, 2014). As reported from Belay (1997), the degree of coiling has a direct relationship with temperature. Besides that, these authors also mentioned mechanical stress during harvesting is another factor causing morphological changes in Arthrospira. These three morphotypes of Arthrospira were already identified by Otago and Kifle (2014) in the same study site including their variability as a response to changes in environmental variables. Similarly, they have been also recognized in other parts of the world including: India (Jeeji Bai and Seshadri, 1980) and Kenya (Kaggwa et al. 2013). However, these authors did not include ultrastructural and molecular aspects that are not well documented in previous studies of Arthrospira. H-type and S-type have significantly larger number of coils than C-type. As the tendency of coiling increases, the number of coils per trichome also increases, which implies the length of trichome also increases. Hence all these measures are positively correlated. Helix pitch has also shown a significant difference among the morphotypes, as it is an indicator of the degree of coiling and therefore could vary between the filaments of H-type, S-type and C-type morphotypes. On the other hand, trichome diameter and helix diameter (in the middle and at the end) did not show any significant differences among the morphotypes. Anyway, these morphotypes are not stable as there could be transformation of one morphotype to another since they are highly dependent on nutrient conditions and environmental parameters (Ballot et al. 2004). They may be hence useful as bioindicators of a mutation of the environmental conditions of medium in cultivation of the natural medium in the lakes where Arthrospira sp. can be found.In support to our finding, Otago and Kifle (2014) and Jeeji Bai and Seshadri (1980) reported that trichome end attenuation is a good parameter for recognizing the morphotypes in addition to helix diameters (in the middle and at the end). Although these and other morphological parameters are regarded as not sufficiently reliable to recognize the taxonomic position of Arthrospira species, they still are in use to distinguish them (Mü-hling , 2000; Thammathorn, 2001; Kaggwa et al. 2013; Rout et al. 2015).
4.2. Growth under Different Concentrations of Ammonium Nitrate
- Nutrient type and concentration are the major limiting factors influencing the growth of microalgae including Arthrospira (Dejsungkranonta et al. 2012; Harwati, 2013). At the beginning of the cultivation not much growth has been observed (Fig. 6). This indicates that Arthrospira, like other prokaryotes, follows different growth phases (Qasim et al. 2012). First a lag phase at the start, is a phase in which they adapt to a new culture medium. After the lag phase a log phase (exponential phase) follows. In this phase cells are growing at higher rate due to optimal growth conditions. The third phase is the stationary phase, in which nutrients start to decline together with the biomass of cells. Finally cells enter the decline (death) phase, in which nutrients are totally depleted and cells are dying at high rate with the exception, in our experiment, of the 0.05 M concentration in which still gradual increments towards the end of our experimental period have been observed. This result could possibly be explained by its inhibitory effect at the beginning of growth due to its concentration and a better adaptation at the end of the growth period. Generally, the best growth of Arthrospira in terms of growth speed, has been observed at 0.03 M NH4NO3 concentration, indicating that Arthrospira requires NH4NO3 at this concentration as an optimal growth medium. This is in agreement with results in similar studies (Nor et al. 2015; Ismaiel et al. 2016), in which a higher concentration of nitrogen sources could have inhibitory effect on the growth of Arthrospira. Nitrogen is also reported as one of the main requirements for Arthrospira anabolism, such as for the synthesis of amino acids (proteins), phycocianin and other cellular components (Uslu et al. 2011). Ammonium nitrate can be used as an alternative nitrogen source for small and large scale commercial cultivation of Arthrospira biomass. It is relatively inexpensive with respect to other nitrogen sources (as NaNO3) and is locally more easily available than other nitrogen sources. It has been preferred not only for being cheaper and easily available but also for its high productivity. Moreover, the different concentrations of ammonium nitrate used could contribute for better understanding of preferred optimal concentrations for growing Arthrospira. Similarly, it has been proven by other studies (Colla et al. 2007; Belay, 2013; Nor et al. 2015; Mashor et al. 2016) that ammonium nitrate is the best alternative cheaper nitrogen source for biomass production of Arthrospira, thanks to its widely distributed use in traditional agriculture. This could also contribute to scale-up Spirulina production with reduced cost.
4.3. Overall Justification, Recommendations and Perspectives
- We planned to address Arthrospira species in three soda lakes of Ethiopia: Chitu, Abijata and Shalla. Unfortunately, we did not find Arthrospira growth from samples of Lake Abijata and Shalla during the successive laboratory cultivation. However, the main aim of this study was to cultivate Arthrospira sp. in tropical conditions in order to evaluate it for possible industrial applications. Therefore, the absence of Arthrospira sp. isolates from the above mentioned sampling sites did not affect the desired goal of the research. Thus, Arthrospira sp. isolates reported in this study were isolated only from samples of Lake Chitu. Reports indicated that Lake Chitu is known for its monoalgal population of Arthrospira fusiformis (Kebede et al. 1994; Kebede 1996). Nonetheless, we should keep in mind the complex taxonomic situation about many species belonging to genus Arthrospira (section 1.2) and in general the difficult delimitation of species in Cyanobacteria (Dvorak et al. 2015).In any case, on the basis of our study, we cannot conclude that other Arthrospira species are absent from Lake Abijata and Lake Shalla for the following reasons: (1) We collected a smaller number of purposive samples targeted for cultivation, (2) part of the sampling points in the lake may not harbor Arthrospira as this cyanobacterum may be distributed in different parts of the lake, (3) they may not survive in the time gap between transportation and cultivation in the lab, and (4) current physicochemical and biological changes in the lake induced by natural (seasonal climatic variation) or anthropogenic activities which could affect the abundance of phytoplankton communities including Arthrospira species. Due to these changes particularly in the Lake Abijata, Arthrospira species are currently largely replaced by non Arthrospira species (Kumssa and Bekele, 2014; Fetahi, 2016). In addition we suggest that a larger number of samples with representative sampling points in each lake during different season needs to be addressed to reach a certain consensus and to avoid confusions about the presence or absence of Arthrospira species in these lakes. The abundance of Arthrospira may vary with seasons of a particular area. This could also help to compare the variability of Arthrospira species in each lake. Arthrospira fusiformis was one of the dominant species of algae in Lake Abijata during 1960 to 1988 (Wood and Thalling, 1988). However, there are no research reports showing the presence of Arthrospira sp. from Lake Shalla even though the water chemistry of the lake and climatic conditions of the location were reported to be suitable for Arthrospira sp. growth (Otago et al. 2014). This partly supports our current report. Generally, tropical soda alkaline lakes are assumed to be hotspots of Arthrospira species and thus further multidisciplinary investigation needs to be carried out to have better understanding of the biology of the lakes including their potential to support the growth of Arthrospira. To further clarify the taxonomic positions and the variability among Arthrospira species of the above mentioned lakes, we suggest assessing large number of strains using morphological, ultrastructural and molecular approaches considering the present work as a baseline data. As the ultrastructural study of Arthrospira sp. of Lake Chitu is not documented previously, further details of ultrastructural features may be required for better understanding the morphological variations within the genus and could be an input to the taxonomic study. Nowadays Spirulina obtain worldwide attentions due to its overall high nutritional quality and quantity of compositions and values. Therefore, small scale and large scale production of Spirulina is required to solve malnutrition problems in developing countries in which the problem is prominent till to date. Besides these, its safety and non toxicity are important characteristics contributing to its acceptability. From this point of view an exact knowledge about the contaminants as those identified in this investigation may be fundamental for assessing the quality of the Spirulina produced locally. There is a need to establish a collaborative research projects between institutions, Non-Governmental Organization (NGO), entrepreneurs and researchers to explore this important natural resource of tropical alkaline lakes such as Lake Chitu for large scale commercial production. This will help to develop various biotechnological applications by Spirulina producing industries. Although a lot of research have been done previously in various aspects of Spirulina of Lake Chitu, from the practical and biotechnological point of view further investigations such as outdoor Spirulina farm and indoor laboratory cultivations are required.
4.4. Conclusions
- Laboratory cultivation of Spirulina under controlled conditions is an important prerequisite which could help to set optimal growth parameters as well as to obtain pure cultures for outdoor or large scale biomass production. This may in turn contribute a lot for malnutrition problems in addition to its economical advantages. From the morphological data of our study it has been confirmed that there are three different morphotypes of Arthrospira belonging most probably to a single species. The morphological parameters described in this study have shown variation among the morphotypes. Since the occurrence of the different morphotypes appear to be related to the environmental parameters of the medium, they may be hence useful as bioindicators of a mutation of the environmental conditions in cultivation of of the natural medium in the lakes where Arthrospira sp. can be found. Further investigation will be necessary to exactly understand the related ecological conditions that lead to specific morphotypes.Spirulina isolates were further grown under different concentrations of ammonium nitrate (alternative nitrogen source) and a relatively low concentration (0.03 M) could be optimum for their growth. In general, from the data of this study it can also be concluded that Lake Chitu is one of the most important natural area to investigate on several aspects of Arthrospira species since this lake appear to be a part of the original distribution area of the species.
ACKNOWLEDGMENTS
- This study was carried out with the financial support of European commission through an Erasmus mundus scholarship programme (TROPIMUNDO). We are greatfull for the general coordinator of the TROPIMUNDO program Farid DAHDOUH-GUEBAS and local coordinators for their great effort and contribution to this programme. We are also grateful to the University of Florence, Laboratory of General Botany for allowing access to use the laboratory including materials. Staff members of the General laboratory of Botany, Corrado Tani and Pietro Di Falco were also greatfull for their unreserved effort and genuine assistance during experimental analysis in the laboratory.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML