-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2018; 8(4): 87-94
doi:10.5923/j.food.20180804.01

Changes in Physicochemical Properties of Table Spreads Produced from African Pear (Dacryodes edulis) Pulp during Storage
Akusu O. M., Wordu G. O., Obiesie C.
Department of Food Science and Technology, Rivers State University, Port Harcourt, Nigeria
Correspondence to: Akusu O. M., Department of Food Science and Technology, Rivers State University, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

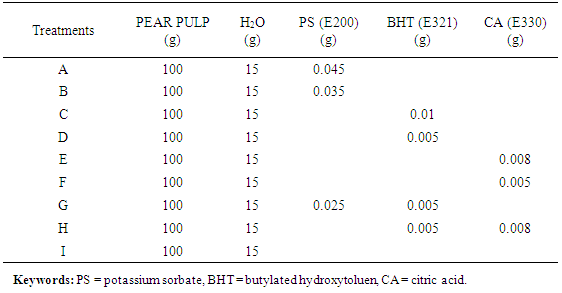
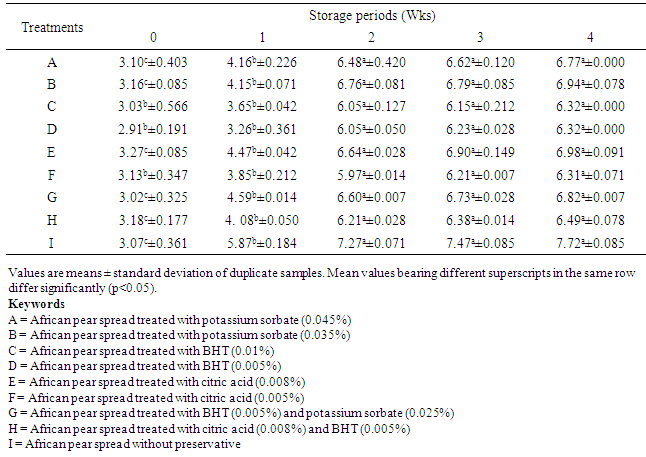
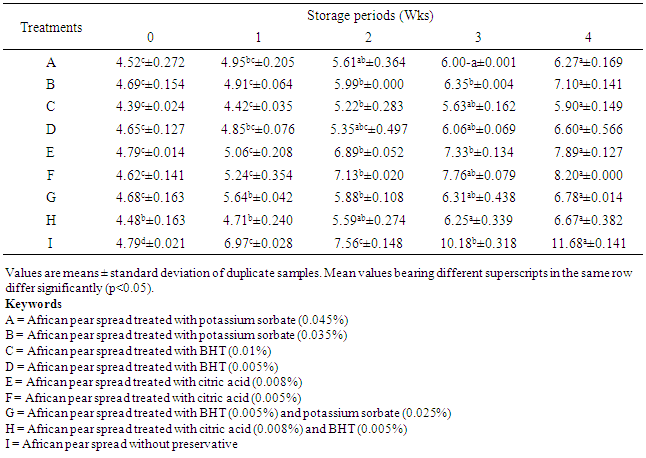
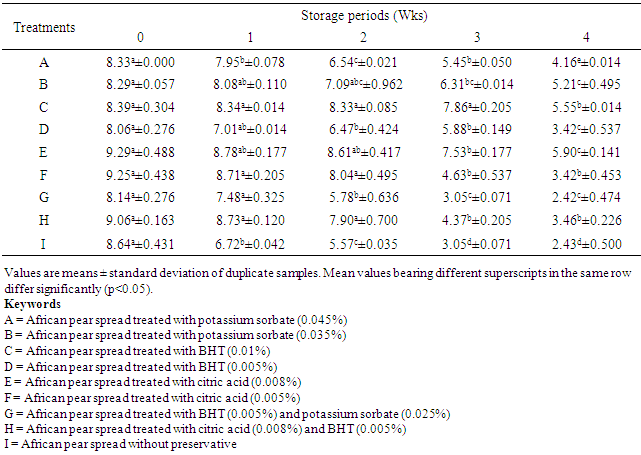
The objective of this study was to evaluate the changes in physicochemical properties of table spread produced from the pulp of African pear (Dacryodes edulis) during storage at room temperature, so as to ascertain its shell stability. The pear pulp was extracted, pasteurized and homogenized to form spreads with different levels of food grade preservatives and tested as such; samples A (with 0.045% potassium sorbate), B (0.035% potassium sorbate), C (0.01% butylated hydroxyl toluene, BHT), D (0.005% BHT), E (0.008% citric acid), F (0.005% citric acid), G (0.025% potassium sorbate and 0.005%, BHT), H (0.005% BHT and 0.008% citric acid) and I (without preservative). The spreads were packed in sealed glass containers and stored at room temperature (28±2°C) for 4 weeks. There was no significant (P>0.05) change in spreadability of the spread samples during storage. However, samples D, H and I showed significant changes in spreadability from weeks 2 to 4. The percentage free fatty acid (FFA) of sample I increased significantly (P<0.05) from 3.07 to 7.72% after 4 weeks of storage, samples C and F which did not show any significant increase in FFA up to end of week 1. The peroxide value of spread samples without preservative increased from 4.79 to 11.68mEq/kg after 4 weeks of storage. Vitamin C content of all samples reduced significantly after 4 weeks of storage. However, Samples C, F, G and H did not show any significant drop in vitamin C content after 1 week of storage, but reduced significantly (P<0.05) from the 2nd to the 4th week of storage at 28±2°C. The pH of sample A reduced significantly from 4.55 to 4.25, sample H reduced from 4.20 to 3.95, while that of sample I reduced from 4.75 to 3.80. There was no significant (P>0.05) change in the individual pH of samples B, C, E, and G during storage at room temperature (28±2°C). The moisture content of samples B, C, and F increased slightly but not significant (P>0.05) during the period of storage. All the spreads treated with preservatives stored better than the untreated sample, spreads treated with 0.010% BHT preserved better than citric acid and potassium sorbate.
Keywords: Physicochemical, Table Spreads, African Pear Pulp, Storage, Preservatives
Cite this paper: Akusu O. M., Wordu G. O., Obiesie C., Changes in Physicochemical Properties of Table Spreads Produced from African Pear (Dacryodes edulis) Pulp during Storage, International Journal of Food Science and Nutrition Engineering, Vol. 8 No. 4, 2018, pp. 87-94. doi: 10.5923/j.food.20180804.01.
Article Outline
1. Introduction
- Vegetable spreads are low fat table spreads when compared to margarine and butter. They are products of nuts and fruit pulps used in various forms, such as paste and slurry [1, 2]. They are used like the commercially available butter and margarine; they can be produced from almond, cashew, hazelnut, macadamia nut, peanut, pecan, pistachio and walnut [3]. Vegetable spread is popular and widely accepted by consumers due to its flavour, good nutritional values and suitability for consumption either alone or in combination with a variety of other foods [4]. African pear pulp has great potential for vegetable spread production. The African pear tree (Dacryodes edulis) is a tropical oleiferous fruit tree that possesses enormous potential in Africa [5]. Various parts of the tree are used in traditional medicine [6, 7]. The wood served for firewood and carpentry [8]. The entire tree is used in Agro-forestry systems for soil conservation [9]. Dacryodes edulis fruit is popular in the diets of many Africans [10]. It can be eaten raw, roasted or boiled in hot water and is eaten alone or used in garnishing cooked or roasted maize [10]. It could also be used as butter to eat bread [7]. Indeed, the pulp of African pear when cooked and seasoned, served as spread (from Cameroon recipe) [10]. According to Ayuku et al. [11], D. edulis has a potential to improve nutrition and food security. During the last three decades, more and more studies have been conducted on D. edulis, essentially the tree and its fruit [10]. The studies on D. edulis focused on the characterization of propagation techniques of D. edulis tree [5], the nutritive value of its pulp and its oil [12], and the oil extraction processes [13]. These studies revealed excellent nutritional qualities of fruit pulp and interesting food processing properties of the oils extracted from the pulp and kernel safou [14]. These have also revealed the importance of this fruit nutritionally, therapeutically and in cosmetic. The pulp, the only edible part of the fruit is particularly rich in lipids, indicated that D. edulis could be an important source of oil [15]. Besides lipids, D. edulis pulp contained substantial amounts of many other nutrients including proteins, carbohydrates, minerals, vitamins and fibres [14]. Sequel to the high content of protein and other essential nutrients in vegetable spreads, attainment of shell stability becomes the manufacturers challenge [16]. Food preservation is the process of treating and handling food to stop or slow spoilage [loss of quality, edibility or nutritional values] and thus allow for longer storage [17]. A principle tenant of food preservation is to maintain the quality and nutritional attributes while preventing spoilage [18]. In general, the fresher the food product, the higher the quality, so the standard of excellence is often freshly prepared products [19]. The retention of vitamin C is often used as an estimate for the overall nutrient retention of food products because it is highly sensitive to oxidation and leaching into water-soluble media during storage, it began to degrade immediately after harvest and degraded steadily during prolonged storage [20]. The degradation of ascorbic acid is known to be affected by pH and changes in pH during storage [21]. pH plays a determinant factor in the quality and stability of juice and fruit based products [22]. Products with low pH will have their shelf life increased [23]. Preservation of vegetable based food products involved preventing the lost in essential nutrients and maintaining desired physicochemical characteristics. Despite the many pathways to deterioration, there are a number of effective food preservatives that have evolved to combat spoilage [19]. Thus, the objective of this study was to evaluate the changes that occur in physicochemical characteristics of table spreads produced from African pear pulp, treated with different levels of preservatives, during storage at room temperature (28±2°C).
2. Materials and Methods
- Mature and good quality fruits from the African pear (Dacryodes edulis) were purchased from the fruit market in Port Harcourt, Nigeria.
2.1. Extraction of African Pear Pulp
- The pulp was extracted using modified traditional method of pear roasting (Figure 1). African pear fruits were sorted and washed with tap water and sodium chloride solution and rinsed thoroughly, roasted at 60°C for 4 min in a hot air oven (model QUB 305010G, Gallenkamp, UK). The roasted fruits were allowed to cool for 10 min, the thin bluish-black epicarp were gently removed and discarded while the soft pulps were scraped off and recovered in sterile stainless steel plates. The extracted pulp was pasteurized by heating at 100°C for 5 min in a stainless steel pot.
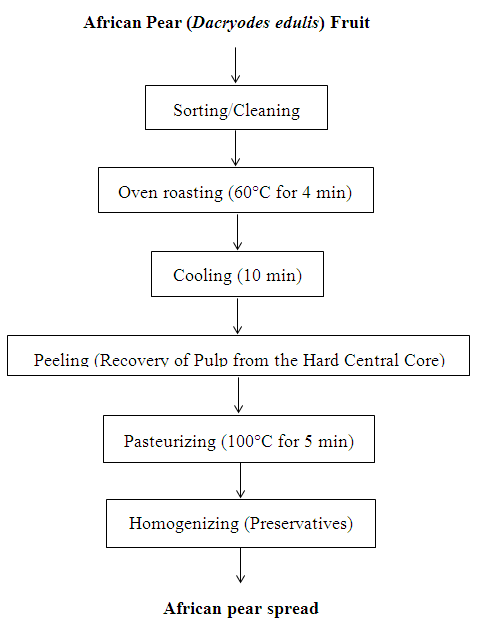 | Figure 1. Flow Chart for the production of African pear spread |
2.1.1. Formulation of African Pear Spread
- The pasteurized pear pulp was treated with different levels of recommended food preservatives [24], as shown in Table 1. The mixture was homogenized properly using a laboratory stirrer (Model JKL 2145, REMI Motors, India). The spreads produced were stored in sealed glass containers at room temperature (28±2°C). Sample were collected at one-week interval for a period of four weeks for storage studies, from zero time, 1, 2, 3 and week 4.
|
2.2. Storage Studies
- Storage stability of the spreads was studied through; changes in spread ability and changes in chemical properties.
2.2.1. Changes in Spreadability of African Pear Spread during Storage
- Spread ability of the African pear spreads at room (28±2°C) was measured using laboratory Bostwick Consistometer (CSC Scientific Company, Inc.), according to the method of Endecotts [25].In the instrument reservoir was placed 100g of pear spread, behind the gate of the consistometer. As the gate is released by pressing the lock release lever, the spring action ensures it opens instantaneously. As the fluid flows down the instrument, its progress was accurately measured using the graduated Bostwick scale. Spreadability (mm/sec) = d/s d = distance covered s = time taken
2.2.2. Changes in Chemical Properties of African Pear Spread during Storage
- Changes in chemical properties of the pear spreads during storage at room temperature (28±2°C) were studied via; changes in peroxide value, percentage free fatty acid, vitamin C content and moisture content, using the AOAC [26] methods.
2.3. Statistical Analysis
- All experiments and analysis were carried out in duplicates and the mean standard deviations values were calculated. Data were subjected to Analysis of Variance (ANOVA), Means were separated using Tukey’s multiple comparison test, and significance accepted at p<0.05 level. The statistical package in Minitab 16 computer program (Software of the State College, Pennsylvania Minitab Inc.) was used.
3. Results and Discussion
3.1. Spread Ability
- Consumer acceptance of spreadable foods depends on their textural characteristics such as spread ability, which is a measure of how easily and uniformly they can be deformed and spread at end-use temperatures [27]. The result for changes in spread ability at ambient temperature (28±2°C) is shown in Figure 2. The spread ability (mm/sec) of samples A, C, E, G, F and J ranged from 0.183 – 0.188, 0.134 – 0.138, 0.231 – 0.238, 0.199 – 0.205, 0.195 – 0.200 and 0.032 – 0.033, respectively, there was also no significant (P>0.05) change in spread ability of these samples during storage. However, samples B, D, H and I showed significant increase in spread ability from zero time to wk 4. The slight increase in spread ability observed in the samples was probably due to oxidative changes that resulted in increased moisture content. None of the samples recorded reduction in spread ability, indicating that table spreads formulated from African pear pulp will maintain good and consistent spread ability on storage.
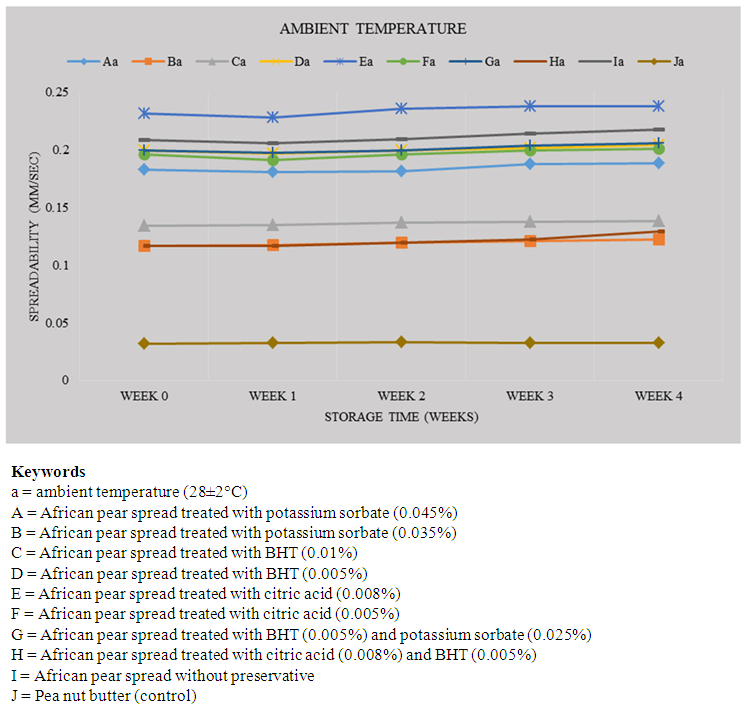 | Figure 2. Changes in Spreadability of African pear spreads treated with different levels of preservatives during storage at 28±2°C |
3.2. Changes in Chemical Properties of African Pear Spreads Treated with Different Levels of Preservatives during Storage
3.2.1. Changes in Percentage Free Fatty Acid (FFA)
- The FFA is the primary quality attribute for edible grade oil/fat. The Purified Food and Adulteration Act specify a maximum acceptable limit of FFA as 3% for milk fats, margarine ≥80% and fat spreads <80% [28]. The unpleasant odour and taste which develops spontaneously in fats, known as rancidity, is of two types: hydrolytic and oxidative [29]. Oxidative rancidity is due to oxidation of double bond of fatty acid with the formation of aldehydes, ketones and acid of lower molecular weight than the fatty acid originally present [29]. The process depends on presence of oxygen; it is hastened by heat light, moisture and certain metal catalysts. Oxidative rancidity is due to chiefly to the oxidation of oleic acid [28]. Changes in FFA of African pear spreads treated with different levels of preservatives and stored at room temperature for four weeks are shown in Table 2. The FFA of sample I increased significantly (P<0.05) from 3.07 to 7.72% after 4 weeks of storage. All other samples showed significant increase in FFA from week 0 – 1, except samples C and F which did not show any significant increase in FFA up to end of week 1. Increase in FFA for each of the individual samples from week 2 – 4 was not significantly different (P>0.05). Increase in FFA is an indication that oxidative break down occurred in the samples during storage at room temperature, this break down was also high after two weeks of storage.
|
3.2.2. Changes in Peroxide Value (PV)
- As shown in Table 3, the PV of sample A increased from 4.52 to 6.27 mEq/kg after 4 weeks of storage. Greater increase in PV was observed in the sample without preservatives, from 4.79 to 11.68 mEq/kg after 4 weeks of storage. Increase in FFA noticed in samples B, C and H after 1 week of storage at room temperature were not significantly different (P>0.05). The PV of the spreads treated with preservatives was all below the maximum acceptable value of 10 mEq/kg for edible vegetable oil [24]. Peroxide value beyond 10 mEq/kg might result in objectionable rancid flavour in fat based products [24]. A rancid taste is often noticeable in many oils when the peroxide value is between 20 and 40 mEq/kg oil [30].
|
3.2.3. Changes in Vitamin C
- Vitamin C (also referred to as L-ascorbic acid) is the lactone 2,3-dienol-L-gluconic acid and it belongs to the water-soluble class of vitamins [31]. It participates in numerous biochemical reactions, suggesting that vitamin C is important for everybody process from bone formation to scar tissue repair [32]. The only established role of the vitamin C appeared to be in curing or preventing scurvy and it is the major water-soluble antioxidant within the body. The retention of vitamin C is often used as an estimate for the overall nutrient retention of food products because it is by far the least stable nutrient; it is highly sensitive to oxidation and leaching into water-soluble media during storage [33, 34]. It begins to degrade immediately after harvest and degrades steadily during prolonged storage [35]. From Table 4, vitamin C content of all samples reduced significantly after 4 weeks of storage. Sample A reduced from 8.33 to 5.21 mg/100g and sample E from 9.29 to 5.90 mg/100g. Reduction in vitamin C content from 7.2 – 2.2 mg/100g and 5.2 – 1.4 mg/100g for pawpaw and watermelon juices, respectively had been reported by earlier study [36]. Samples B, C, E, F and H did not show any significant (P>0.05) drop in vitamin C after 2 wks of storage. The result showed that vitamin C retention was enhanced by treatment with BHT, potassium sorbate and citric acid. The stability of vitamin C by higher acid level of citric acid was reported by Padayatty et al. [37].
|
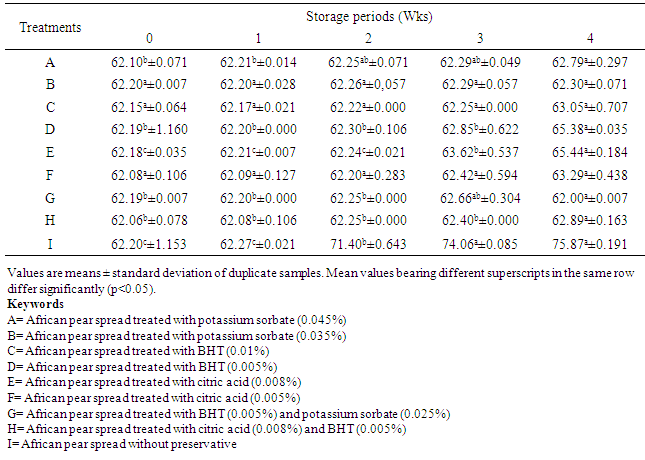
3.2.4. Changes in pH
- Results for changes in pH of African pear spreads during storage are shown in Table 5. The pH of sample A reduced significantly from 4.55 to 4.25, sample H reduced from 4.20 to 3.95, while that of sample I reduced from 4.75 to 3.80. There was no significant (P>0.05) change in the individual pH of samples B, C, E, and G during storage at room temperature (28±2°C). Reduction in pH of other fruit juices (pineapple, watermelon and pawpaw) from 4.4 – 3.3, 5.8 – 4.5 and 5.4 – 4.0, respectively, during storage at four weeks had been reported earlier [36]. Reduction in pH during storage is probably due to oxidative changes that result in increased free fatty acid content of the product.
|
3.2.5. Changes in Moisture Content
- The moisture content of table spreads produced from African pear pulp during storage is given in Table 6, the moisture content of sample I increased significantly (P<0.05) from 62.20 to 75.87% after 4 weeks of storage. The moisture content of samples B, C, and F increased slightly but not significant (P>0.05) during the period of storage.
|
4. Conclusions
- The findings from this study showed that table spreads produced from African pear pulp and treated with 0.010% BHT preserved better than citric acid and potassium sorbate. Increase in PV (6.27 – 7.89 mEq/kg) still fall below the maximum acceptable value of 10 mEq/kg for edible vegetable oil. All the spreads treated with preservatives stored better than the untreated sample, and maintain good and consistent spreadability during the period of storage.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML