-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2018; 8(3): 53-59
doi:10.5923/j.food.20180803.01

A Novel Processing Method for Bamboo Shoots in India – ‘Cluster Level Bamboo Shoots Processing’
Deepti Dabas
PhD Food Science, Pennsylvania State University, USA
Correspondence to: Deepti Dabas, PhD Food Science, Pennsylvania State University, USA.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Bamboo is an evergreen plant with multiple uses including as edible bamboo shoots. Despite having a large area under bamboo cultivation, India exports only a small quantity of bamboo shoots. This is due to limited processing facilities around shoot growing areas. Shoots were processed using ‘cluster level shoot processing’ which is suitable for the remote regions of Northeast India. The aim of this study was to examine the effect of storage at 37°C on processed shoots for 14 days. The shoots’ microbiological quality improved as time went on; salmonella and shigella which were present at the beginning, were no longer detected on day 4 and 7, respectively. The pH reduced during the storage period –benzoic acid was detected in the shoots, which may have improved the microbiological quality of the shoots. This technology can be utilized to tap the unutilized bamboo shoot sector of the country.
Keywords: Bamboo shoots, Processing, Microbiological changes, Chemical changes, Dendrocalamus asper
Cite this paper: Deepti Dabas, A Novel Processing Method for Bamboo Shoots in India – ‘Cluster Level Bamboo Shoots Processing’, International Journal of Food Science and Nutrition Engineering, Vol. 8 No. 3, 2018, pp. 53-59. doi: 10.5923/j.food.20180803.01.
1. Introduction
- Bamboo are a versatile and evergreen group of plants which are grown in many continents including Asia. Bamboo is a grass belonging to family Poaceae and is spread over 1,250 species and 75 genera over the world. Out of these, about 136 species under 23 genera are found only in India [1].Bamboo has multiple uses; for production of various bamboo based panels – flooring, pulp, charcoal production, handicrafts and as edible shoots [2]. Due to its excellent strength bamboo culm is used for making furniture, handicrafts, containers, tool handles, poles, musical instruments, bows and arrows, boats, rafts, fishing poles [2]. The rhizome produces culms on the surface and roots underneath [2]. Bamboo grows quite densely producing multiple culms – also it is a fast grower [2]. These attributes enable bamboo to be useful in water conservation, soil restoration and protection and carbon sequestration [2]. It is estimated that global bamboo market is at $12000 million of which India has a mere share of 4.5% despite having 31.1% of total bamboo growing area in the world [2]. In India, out of this total bamboo growing area, around 28% of area is in North East India. This area is responsible for providing 66% of bamboo resources in the country [3]. North East part of the country has a climate conducive to bamboo growth and thus almost all the states in this region have bamboo growing in forest [2]. These states register high rainfall and hence high humidity and optimum temperatures – conditions which favor bamboo growth [2].Bamboo shoots are a delicacy in many countries including China, Taiwan, Thailand, Vietnam, Philippines, India and Japan [4]. Between December 2016 and December 2017, around 61 million Kg of canned bamboo shoots were imported into the USA from different countries: China, Thailand, Taiwan, Japan, and India – with China being the biggest player [5]. This is despite India having a larger area under bamboo cultivation than China [3].Shoots are present in mostly forest area in India some of which are not near habitation or may not allow access for timely harvesting and processing. The shoots are typically harvested 7-14 days after the emergence when the shoot height is about 15-30 cm depending on the species [2]. If the shoots are not harvested soon enough after they start to sprout out of the ground, these shoots/young culms can turn into woody culms. After harvest, due to their high-water content and the inherent enzyme activity, the bamboo shoots start to degrade soon after if they are not processed [6]. Due to lack of processing facilities in the forest areas in which they commonly grow, accompanied by high humidity and temperatures of these areas, the shoots spoil very fast after harvesting. Also, fresh shoots are only available in some regions in the country from May-September which means their availability is limited. Hence, they are not exploited as much as they could be and there is a need of processing facilities that do not require lot of investment [2].Various traditional processing techniques can be used to preserve bamboo shoots. Vacuum processing and canning are two common processing methods used industrially. However, both these processes require elaborate processing plants which requires considerable investment. These types of shoot processing facilities are not commonly found in the remote regions of North East India and other shoot growing regions [2]. Other common traditionally used processing methods are fermentation and drying which are able to preserve the shoots for longer time in the absence of any elaborate processing machinery. However, both these processes change the composition, taste, flavor and characteristics of the bamboo shoots and hence are not suitable if the bamboo shoots are intended to be preserved in their fresh form [1].In order to tap the bamboo shoots to their full potential, methodologies which can take existing limitations into consideration need to be developed. Hence there is a need of processes which can minimally process bamboo shoots just after harvesting so that minimum equipment and space are enough. At the same time bamboo shoots need to preserve for enough time during which they can be transported so that they can be moved to the facility where long term preservation can occur [2]. A simple shoot processing had been developed by us at The North East Centre for Technology Application and Research (NECTAR) in India which would keep shoots fresh for 7-10 days if water is used and longer (up to 20 days) if salt is added to the shoots [2]. This facility requires around 150 ft2 of area and is called ‘cluster level shoot processing’. In simplest form, shoots can be peeled, washed, soaked overnight & drained and packaged in flexible nylon based pouches along with water. Processed bamboo shoots using this method can not only be easily stored and transported but can bring a higher price due to their hygienic handling and ability to preserve for longer time period compared to fresh shoots. Packaging of the shoots using this method retains their flavor, texture and freshness for a longer period and reduces wastage and spoilage. These shoots can be transported to processing sites or to markets where better long-term processing methods can be used. They can also be sold in other markets for direct consumption [2]. The temperatures in these regions can vary from 20 to 35°C depending on the area and time of the year [7]. Having a cold chain would be beneficial to keep the product preserved longer but is not common in many states of North East India including Meghalaya and Arunachal Pradesh [8]. Hence the product should be used as early as possible.Other studies have investigated minimal processing of bamboo shoots. Low temperature and packaging have been used to reduce transpiration losses occurring in bamboo shoots stored in the open and at prevalent temperatures (which can range from 20- 30°C). Discoloration is the major cause of quality loss of shoots [9]. Fungicides have also been suggested to preserve the shelf-life. Wang and He (1989) reported that addition of fungicide to bamboo shoots packaged in polyethylene film extended their shelf life to 62 days at 0°C [6]. Kleinhenz et al. (2000), attempted a combination of different packaging and low temperatures to increase the shelf-life of bamboo shoots – poly vinyl chloride (PVC) gave the best results followed by low density poly ethylene (LDPE) and then micro perforated LDPE. They were able to get a shelf-life of 28 days when kept at 1-2°C [9]. Shoots were packaged in modified atmospheric packaging (MAP) in 0.04 mm polyethylene pouches and stored under refrigerated conditions. Compared to control (shoots in polyethylene but not sealed; kept refrigerated), the MAP preserved shoots performed better and did not show discoloration [10].This study aimed at using this cluster level shoot processing method to preserve shoots of the species Dendrocalamus asper. Dendrocalamus asper is very commonly used for food and grows in many countries including Thailand and Philippines[11]. Shoots were stored at 37°C and their chemical and microbiological changes were evaluated over a period of 14 days.
2. Material and Methods
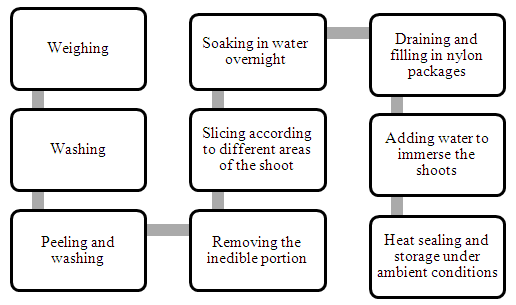
- Materials: Dendrocalamus asper was harvested during August from plantation located in Garh Mukteshwar (Hapur, Uttar Pradesh) in India. It was kept at 4°C until used. All reagents and solvents used were of highest commercial grade (Sigma, India).‘Cluster level bamboo shoot processing’Bamboo shoots were processed using the process as described in the flow chart below. The shoots were washed and peeled and then soaked in water overnight, at room temperature. Next day, water was drained and shoots were sliced. The shoots were then placed in 90 micron thick nylon pouches to which water was added and these packs were heat sealed. These processed shoots were incubated at 37°C and different packages were opened on specific days to analyze the chemical and microbiological parameters. A control to which no water was added while packaging, was also prepared alongside and kept at 37°C. This was compared visually to the processed shoots during the duration of storage.
 Methods of analysisMicrobiological analysisAerobic Plate Count (APC) were estimated as explained in Bacteriological Analytical Manual [12]. E coli and coliforms were determined using Bacteriological Analytical Manual [13]. To detect Salmonella and Shigella methods as stated in Bacteriological Analytical Manual were referred to [14, 15].Chemical analysisSalt content was analyzed using AOAC 937.07 [16]. Brix content was estimated using AOAC 932.14 [17]. Acidity was measured using AOAC 925.34 [16]. pH was analyzed using AOAC 981.12 and acidity using AOAC 942.15 [16]. Benzoic acid was estimated using AOAC 963.19 [16].Determination of hydrocyanic acid25 mg of cut bamboo shoot was ground and placed in flat bottom bottle. Immediately, 0.5 mL of 0.1 M pH 6.0 phosphate buffer was added. After slow mixing, yellow picrate paper was attached to the plastic strip and was placed in bottle, after which the bottle was closed. It was allowed to stand for 16-24 h at room temperature. After this the orange-brown picrate paper was immersed in 5.0 mL of distilled water for 30 min with occasional gentle shaking. Absorbance of solution was measured at 510 nm and the amount of hydrocyanic acid calculated [18].Statistical analysis. Three independent samples were used to obtain data. Statistical analysis was carried out by estimating mean and standard deviation. Pairwise comparison test was then carried out to find if the differences are significant. A p value of (<0.01) was used to determine significant differences.
Methods of analysisMicrobiological analysisAerobic Plate Count (APC) were estimated as explained in Bacteriological Analytical Manual [12]. E coli and coliforms were determined using Bacteriological Analytical Manual [13]. To detect Salmonella and Shigella methods as stated in Bacteriological Analytical Manual were referred to [14, 15].Chemical analysisSalt content was analyzed using AOAC 937.07 [16]. Brix content was estimated using AOAC 932.14 [17]. Acidity was measured using AOAC 925.34 [16]. pH was analyzed using AOAC 981.12 and acidity using AOAC 942.15 [16]. Benzoic acid was estimated using AOAC 963.19 [16].Determination of hydrocyanic acid25 mg of cut bamboo shoot was ground and placed in flat bottom bottle. Immediately, 0.5 mL of 0.1 M pH 6.0 phosphate buffer was added. After slow mixing, yellow picrate paper was attached to the plastic strip and was placed in bottle, after which the bottle was closed. It was allowed to stand for 16-24 h at room temperature. After this the orange-brown picrate paper was immersed in 5.0 mL of distilled water for 30 min with occasional gentle shaking. Absorbance of solution was measured at 510 nm and the amount of hydrocyanic acid calculated [18].Statistical analysis. Three independent samples were used to obtain data. Statistical analysis was carried out by estimating mean and standard deviation. Pairwise comparison test was then carried out to find if the differences are significant. A p value of (<0.01) was used to determine significant differences.3. Results
- Microbiological changesThe Aerobic plate counts (APC) were higher in fresh shoots then in processed shoots. APC decreased significantly at each time point except from day 10 to 12 (Table 1). Fresh shoots also showed presence of coliforms which were not detected from day 4 onwards. Salmonella and Shigella were present at the beginning but were not detected during storage at 37°C after 4 and 7 days, respectively and onwards (Table 1).
|
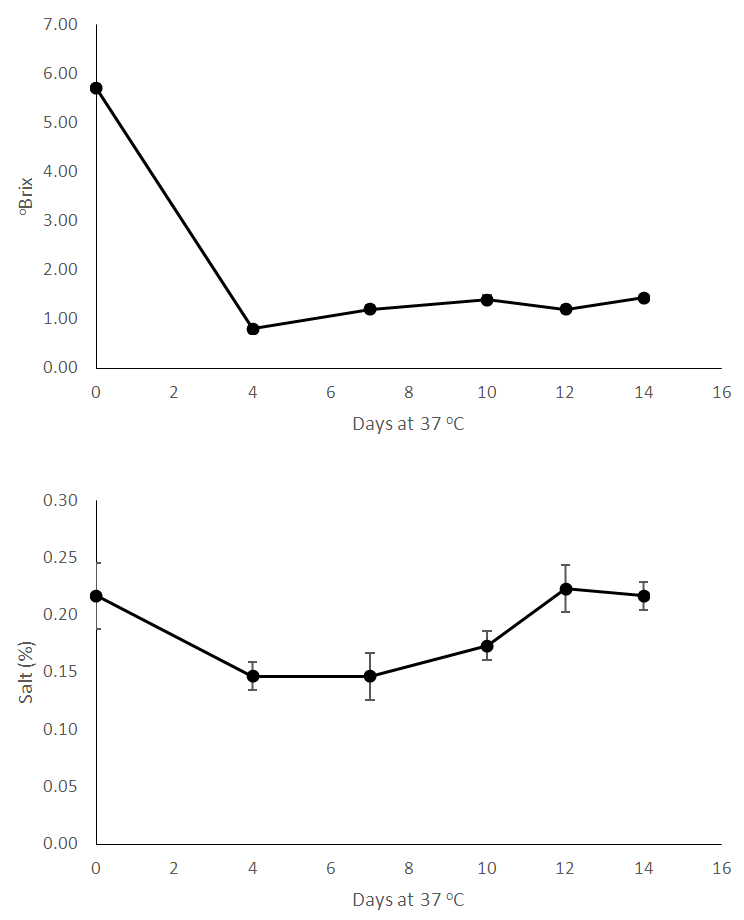 | Figure 1. Chemical analyses of processed bamboo shoots during storage for 14 days – oBrix and Salt content |
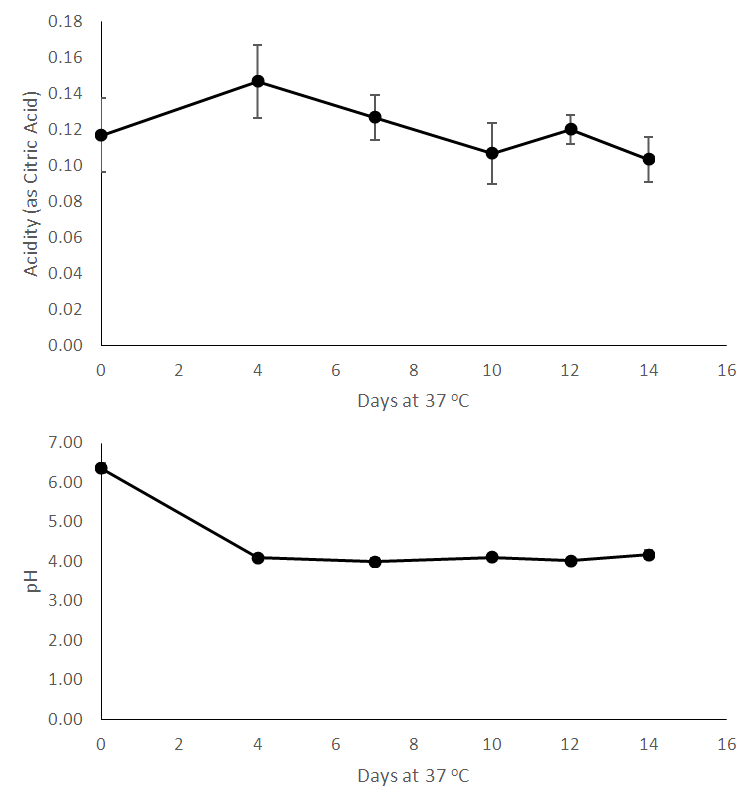 | Figure 2. Chemical analyses of processed bamboo shoots during storage for 14 days – Acidity (as citric acid, %) and pH |
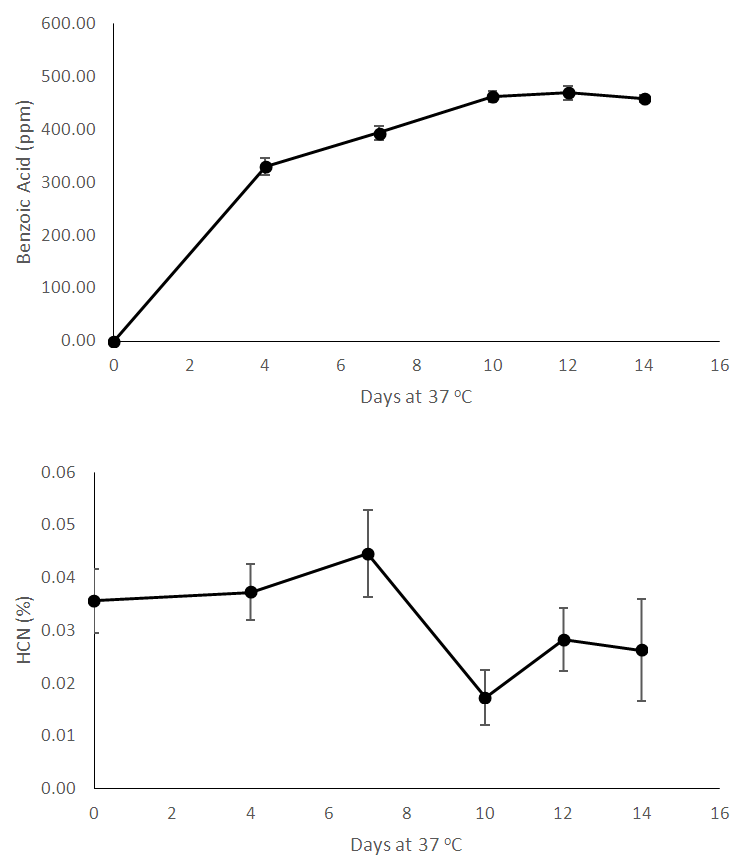 | Figure 3. Chemical analyses of processed bamboo shoots during storage for 14 days – Benzoic Acid (ppm) and Hydrocyanic Acid content (%) |
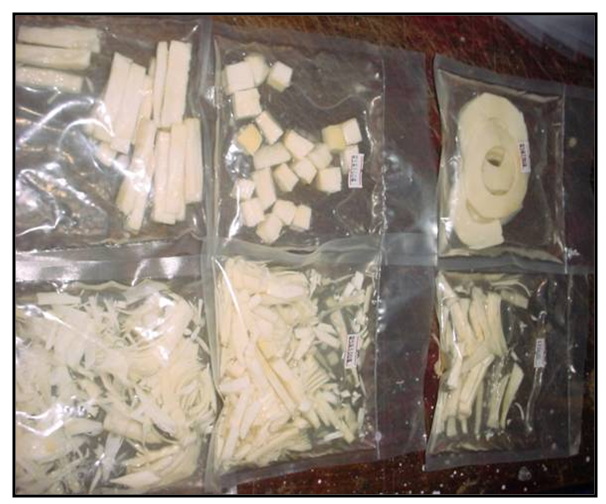 | Picture 1. Bamboo shoots processed using cluster level bamboo shoots processing |
 | Picture 2. Equipment for ‘cluster level bamboo shoot processing’ |
4. Discussion
- This study described a simple technology which can make the bamboo shoot available for further processing. There is a clear demand for bamboo shoots internationally and also in the country which can be met by adopting to this type of minimal processing. The chemical and microbiological changes were tracked in the shoots in order to better understand the ongoing changes. As the shoots were stored, reduction in soluble solids occurred which may occur due to dissolution into surrounding water. This phenomenon should be studied in more detail. As the time went on, the pH reduced but acidity (measured as citric acid) did not show significant changes, therefore we analyzed the shoots for presence of benzoic acid. Bamboo shoots have been found to contain benzoic acid [19]. Bamboo leaves have also found to contain benzoic acid [20]. However, we were not able to detect benzoic acid in fresh shoots – although after processing, the shoots demonstrated the presence of benzoic acid. As indicated by reduction in microbial count, the overall microbiological quality of shoots improved during storage. Benzoic acid is known to be anti-micorbial [21] and may be responsible for improved microbiological quality of stored shoots. Hydrocyanic acid content did not show significant changes during storage. Heat treatment like boiling helps to reduce hydrocyanic acid [11] and these shoots would require a heating step to reduce hydrocyanic acid.We hypothesize that the reason for better quality of shoots soaked in water compared to control is the lack of contact with oxygen – this enables to arrest enzyme activity and other catalytic changes which happen in shoots without any water (control) – these shoots are in direct contact with air and hence do not stay fresh for long. More research should be carried out to better understand this phenomenon.This processing method can make shoots available to processors or even directly to consumers who prefer fresh and safe food options. Today’s customers want their foods to be least processed, devoid of any artificial ingredients and be as clean label as possible. This type of processing gives consumers that option along with the convenience of getting ready-to-cook bamboo shoots. More research should be carried out on technical, commercial and sustainable merits of this technology.
ACKNOWLEDGEMENTS
- I acknowledge the funding from NECTAR for this work. I also acknowledge the support received from Robin Boyle for statistical analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML