-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2018; 8(2): 15-26
doi:10.5923/j.food.20180802.01

The Impact of Post-Harvest Traditional Technologies on Nutritional Value and Antioxidant Activity of Seeds Kernels “akpi” of Côte d’Ivoire (West Africa)
Mamadou Coulibaly1, Denis Yao N’dri1, Verdier Abouo1, Camille Adam Kouamé1, 2, Nestor Kouakou Kouassi1, Georges N’guessan Amani1
1Food Biochemistry and Tropical Products Technology Laboratory, Biochemistry and Food Technology Section, Department of Food Science and Technology, University Nangui Abrogoua, Abidjan, Cote d’Ivoire
2Food Biochemistry and Tropical Products Technology Laboratory, Nutrition Section, Department of Food Science and Technology, University Nangui Abrogoua, Abidjan, Cote D’Ivoire
Correspondence to: Camille Adam Kouamé, Food Biochemistry and Tropical Products Technology Laboratory, Biochemistry and Food Technology Section, Department of Food Science and Technology, University Nangui Abrogoua, Abidjan, Cote d’Ivoire.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Proximate composition (moisture, protein, total dietary fibre, lipids, ash, phenolic compounds, acidity and pH), minerals (K, Ca, Mg, P, Cl and K) and trace elements (Fe, Zn) were determined in Ricinodendron heudelotii (Baill.) Pierre ex Pax seeds kernels found in six producing areas of Côte d’Ivoire (West Africa). There were significant differences between the Ricinodendron heudelotii seeds kernels samples for all the parameters studied; in addition, both the locality of production and the Ricinodendron heudelotii seeds kernels extraction systems showed a noteworthy influence on the mineral and element composition of the seeds kernels. The seeds kernels from Ricinodendron heudelotii were rich in lipids (47.8-54.8%), protein (23.4-28.3%) and energy (159.2-267.0 kcal/100g). Phosphorus (1.8-2.3%) was the predominant mineral. Polyphenols (54.56-156.8 mg/100g) and oxalates (382.6-593.3 mg/100g) were the main phytochemical compounds identified. It is worth mentioning that, the consumption of seeds kernels may contribute relatively high intake levels of lipids, protein, phenolic compounds, mineral and micronutrient (K, Mg, Ca, Mn, Fe and Zn). Application of multidimensional scaling (MDS) correctly classified the seeds kernels from Ricinodendron heudelotii according to the kernels extraction into four extraction system: Agboville1-Bondoukou diagram, Agboville2-Vavoua diagram, Divo diagram and Lakota diagram.
Keywords: Ricinodendron heudelotii, Traditional extraction method, Nutritive value, Antioxidant properties, Seeds kernels, Multidimensional scaling
Cite this paper: Mamadou Coulibaly, Denis Yao N’dri, Verdier Abouo, Camille Adam Kouamé, Nestor Kouakou Kouassi, Georges N’guessan Amani, The Impact of Post-Harvest Traditional Technologies on Nutritional Value and Antioxidant Activity of Seeds Kernels “akpi” of Côte d’Ivoire (West Africa), International Journal of Food Science and Nutrition Engineering, Vol. 8 No. 2, 2018, pp. 15-26. doi: 10.5923/j.food.20180802.01.
Article Outline
1. Introduction
- In Africa and Cote d’Ivoire (West Africa) in particular, several wild fruits and vegetables are consumed among the species of non-timber forest products (NTFPs) [1]. These wild fruits and vegetable contribute significantly to meeting rural nutritional needs and a source of household income [2]. Notable among these wild fruits and vegetables is the Ricinodendron heudelotii (Baill. Pierre ex Pax) a member of the Euphorbiaceae family. The plant is known to have two subspecies, namely, heudelotii and africanum (Müll. Arg.) [3]. Subspecies heudelotii is known to be common in Senegal and Benin, while the subspecies africanum is predominant in the southern part of Nigeria and South Africa. The subspecies R. heudelotti is the only one found in Côte d'Ivoire. Ricinodendron heudelotii (R. heudelotti) specie is a fast-growing tree, reaching up to 50 m in height and 2.7 m in girth or a diameter of 150 cm, which grows in the world’s tropical area. In Sub-Saharan Africa, it is one of the main trees of the tropical forest in the equatorial region. The tree has several local names such as “akpi” in Côte d’Ivoire, “Njansang” in Cameroon, “Okwe” in south east Nigeria, “Bomoko” in Central Africa Republic, and “Betratra” in Madagascar [4]. The edible part of the fruit (kernel) is rich in lipids (47.8 - 50%) but also contains proteins (22.38 - 24.91%), phosphorus (1693 mg/100 g), calcium (1013 mg/100 g), potassium (811.4 mg/100 g) and magnesium (528.6 mg/100 g) [4, 5]. Several kernel extraction technologies exist independently of production areas. Kernels from R. heudelotii fruits are extracted depending on the capacity of the women. Generally, drupes that fall-down from the trees are piled and left to ferment for two weeks or more to enable the pulp to rot. Once rotten, the seeds can be extracted by washing and boiling, and are then put into cold water and left for 24 hours. They are then subjected to further boiling, which cracks the seed shells exposing the kernels for extraction by knife or any other sharp object. The kernels are then dried in the sun or in an oven. Once dried, these kernels can be stored for several years and may be sold throughout the year in urban markets [6]. The seeds of «Akpi» (Ricinodendron heudelotii), because of its very pronounced and appreciated aroma, are extensively used in many diets in Africa, more specifically in Côte D’Ivoire. Usually, the kernels of the plant are used in African soups as an additive function (e.g. as a thickener) and taste lifter [7]. Studies on post-harvest and processing technologies for fruits, nuts and kernels have shown that, the chemical and nutritional property are one of the factors of food variation that constitutes the kernel extraction systems [8]. Despite the increasing number of scientific reports on the R. heudelotti [9-11], studies on the overall nutritional quality of seeds kernels from Côte d’Ivoire are few. With the aim of defatted producing defatted flours, knowledge of its nutrients and some anti-nutrients composition with respect to traditional extraction process need to be generated. The objectives of this study therefore, was to investigate the nutrients, minerals, phytochemical and bioavailability of flours from R. heudelotti (Baill.) Pierre ex Pax seeds kernels found in six producing areas of Côte d’Ivoire. In this study, the effect of traditional kernels extraction process on the physico-chemical and antioxidant properties of kernels flours from R. heudelotti was assessed.
2. Materials and Methods
2.1. Materials Chemicals and Reagents
- All chemicals and reagents used were of analytical grade and purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ultra-pure water with a resistivity of 18.2 MΩ was used in all experiments provided by ELGA (Flex III, U.K) water purification system.
2.2. Sampling and Sample Preparation
- The plant material was mainly the kernels of R. heudilotii (Figure 1).
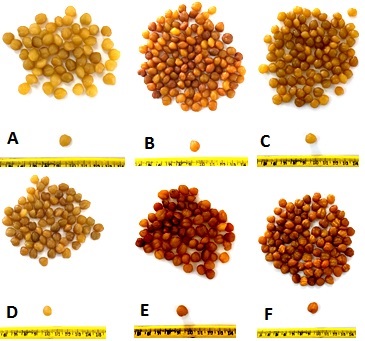 | Figure 1. R. heudelotii seeds kernels samples “Bondoukou” (A), “Agboville2”, (B), “Divo” (C), “Lakota” (D), “Vavoua ” (E) and “Agboville1” (F) |
2.3. Proximate Analysis
- The methods used for sample treatment and analysis (Moisture, ash, lipids, protein) were carried out following standard procedures recommended by AOAC [12]. Moisture was determined by gravimetric method, heating in an oven at 105±1°C until constant mass. Total nitrogen was determined by the Kjeldahl method and converted into protein, using factor 6.25. Total lipids were extracted by the Soxhlet technique with hot solvent (hexan) and afterwards were determined by gravimetric. Ash was determined by gravimetric of incinerated sample, in muffle, at 550°C. The total carbohydrates (TCHO) were calculated by difference as suggested by FAO/WHO procedure [13]. The total dietary fiber content was determined following Prosky’s protocol [14]. Total sugars (TS) were determined using the by the phenol-sulphuric acid method [15] while the reducing sugars were quantified by the oxido-reduction method using 3.5-dinitrosalysislic acid (DNS) as oxidizing agent [16]. A reaction mixture was prepared by adding 0.1 ml of sample, 0.9 ml of ultra-pure water and 0.5 ml of DNS. The mixture was heated to the water-bath 100°C for 5 min and then cooled down to room temperature and diluted with distilled water to 3.5 mL. After rapid cooling during 30 min, the absorbance was recorded at 480 nm. As for total sugars, 0.1 ml from the sample was added to 0.9 ml distilled water and then 1 ml phenol [5% (p/v)] and 1 ml H2SO4 extract were added. After the softly homogenized, the mixture was heated to the water-bath 100°C for 15 min. The absorbance was measured at 540 nm. The energy content of the sample was computed from the proximate data using the Atwater formula. It was estimated by multiplying each gram of carbohydrate, protein and lipid by 4 kcal, 4 kcal and 9 kcal respectively [17]. All analyses were performed in triplicate.
2.4. Mineral Composition
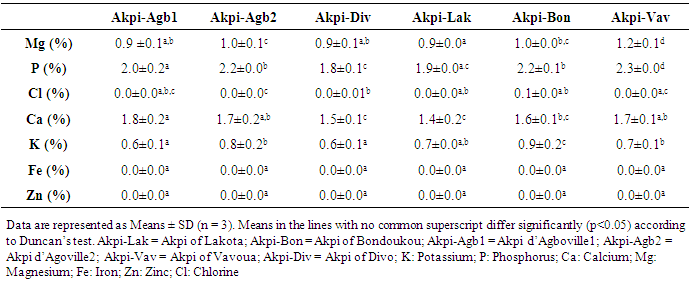
- The sample was accurately weighted and dry-ashed (550°C, one night; method 40–70.01) [18] in a muffle furnace (Cavallo Srl, Buccinasco, Italy). Grey ashes were treated with high purity hydrogen peroxide (H2O2 30% Suprapur, St. Louis, MO, USA) to obtain white ashes, that were dissolved with acid solution (2 mL HCl 30% Suprapur, St. Louis, MO, USA) and diluted with distilled water in volumetric flasks. Mineral concentrations (potassium (K), magnesium (Mg), iron (Fe), calcium (Ca) and zinc (Zn)) were determined by Atomic Absorption (Analyst 800 Perkin Elmer, Waltham, MA, USA), while phosphorous (P) - and Chlorine (Cl) were determined by a colorimetric method using Cary 3E UV-VIS Spectrophotometer (Varian, Mulgrave, Australia) [19]. All analyses were carried out in triplicate with two measurements per analysis. All minerals were reported as mean and standard deviation; data are expressed as% dry weight.
2.5. pH Determination
- Ten grams of powdered sample was diluted with 90 ml distilled water and mixed thoroughly and the pH of the sample was measured using a pH meter model 744 (Metrohm AG, Herisau, Switzerland) according to the procedure described in the Swiss Food Manual (Schweizerisches Lebensmittel-Buch, 2001). The pH meter was calibrated with standard buffers 4 and 7, before measuring the pH of the mixture.
2.6. Total Titrable Acidity (TTA)
- The acidity of kernels was determined by titration following the method described by AOAC [12] using phenolphthalein (Sigma–Aldrich Chemical Co., St. Louis, MO, USA) as an indicator. The acidity of the samples was calculated by using the following equation
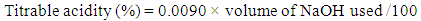 | (1) |
2.7. Phytochemical Analysis
2.7.1. Total Flavonoids Determination
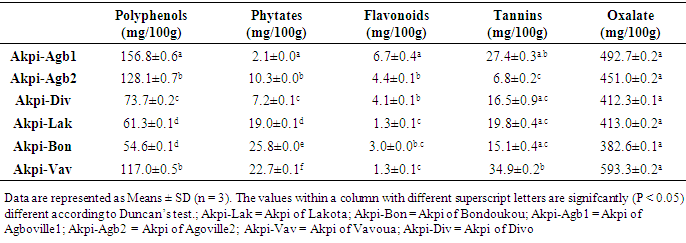
- Total flavonoid content was determined using a colorimetric assay as described by the method of Meda et al. [20]. Sample extracts were evaporated to dryness and re-dissolved in 80% ethanol to be ready for the analytical test. Briefly, 1 mL of a sample (ethanolic solutions or R. heudilotii kernels extract) was mixed with 3 mL 95% ethanol (v/v), 0.2 mL 10% aluminum chloride (m/V), 0.2 mL of 1 mol/L potassium acetate and 5.6 mL water. A volume of 10% (m/V) aluminum chloride was substituted by the same volume of distilled water and used as a blank. After incubation at room temperature for 30 minutes, the absorbance was measured at 415 nm using a UVVIS spectrophotometer (Cary 50 Bio, Varian Australia Pty. Ltd., Victoria, Australia). Quercetin (Sigma, St. Louis, MO, USA) was used to perform the calibration curve (standard solutions of 6.25, 12.5, 25.0, 50.0, 80.0 and 100.0 µg/mL) in 80% ethanol (V/V). Flavonoids in R. heudilotii seeds kernels extracts were expressed as mg quercetin equivalents per gram of dried sample (mg QE/g).
2.7.2. Phytic Acid Content
- Phytic acid was determined by the New Chromophore method of Mohammed et al. [21]. Briefly, a mass of 0.5 g of the sample is homogenized in 25 mL of 3% trichloroacetic acid (TCA) for 45 min and then centrifuged at 3500 rpm for 15 min. To five (5) mL of the supernatant obtained are added 3 mL of 1% iron chloride prepared in hydrochloric acid HCl (1 N) and then heated on a water bath for 45 min. After cooling the mixture, 5 mL of hydrochloric acid (HCl) are added and the mixture is then left to stand for 2 hours. Five (5) mL of 1.5 N sodium hydroxide are then added to the mixture obtained above; the whole is carried on a water bath for 15 min and centrifuged again at 3500 rpm for 15 min after cooling. One milliliter of the supernatant is removed, to which is added 4.5 mL of distilled water, 4.5 mL of ortho-phenanthroline reagent. The optical density is read at 470 nm on the spectrophotometer against a blank. The amount of phytate content were expressed as mg/100 g dry weight.
2.7.3. Tannins
- Tannins were determined using acidified vanillin and (+) catechin as standard [22]. To determine tannins in R. heudelotti seeds kernels two grams of sample were mixed with 30 mL acetone 80%. The mixture was stirred for 15 min and filtered under pressure. Acetone was separated using a rotavapor (Buchi, R124) and then mixed with freshly prepared 4% vanillin in ethanol. The mixture were stirred, treated with concentrated HCl and quantified by spectrophotometry (PG Instruments, England) at 500 nm. Tannins content of samples was estimated using a calibration curve for (+)-catechin. The results obtained were expressed as mg catechin equivalent/g of sample, on a dry weight basis.
2.7.4. Oxalates
- Oxalates content was determined by using the method described by Day and Underwood [23]. Briefly, one gram of dried powdered was weighed into 100 mL conical flask. 75 mL of 1.5 N H2SO4 was added and the solution was carefully stirred intermittently with a magnetic stirrer for about 1 h and then filtered using Whatman no1 filter paper. 25 mL of sample extract (filtrate) was collected and titrated hot (80-90°C) against 0.1 N KMnO4 solution to the point when a faint pink colour appeared that persisted for at least 30 sec. The results were expressed as mg/100 g dry weight.
2.7.5. Total Phenolic Assay
- The phenolic compounds were extracted following the procedure described by N’Dri et al. [24], and determined by the Folin-Ciocalteu assay [25]. Briefly, a dried sample (1 g) was extracted with 10 mL of 80% methanol at room temperature and then reacted with a 10-fold diluted Folin-Ciocalteau reagent. Sodium carbonate at a concentration of 6% (w/v) was added, and the final volume was made up with deionized water. After incubation at room temperature for 15 min, the mixture’s absorbance was measured against the gallic acid standard (the blank being prepared under the same conditions as before but without extract) at 725 nm using a spectrophotometer (PG Instruments, England). Total phenolic content was expressed as mg gallic acid equivalent (GAE)/100 g sample.
2.8. Statistical Analyses
- The data obtained were subjected to statistical analysis performed with Statistica 9.0 (StatSoft, Krakow, Poland). They were recorded as means ± standard deviation (SD), and analysed by Excel 2013. Analysis of variance (ANOVA) were used to study the differences between samples. Duncan’s multiple range test (p <.05) was used to determine the significances within treatments. The similarity between extraction systems of the seeds kernels were examined by multidimensional scaling (MDS). A proximity matrix was required to perform multidimensional scaling (MDS), which is a collection of similarity estimates between each pair of samples in the set of chemical constituents. Proximity was determined by simple Euclidean distance applied to standardized data. Similarity groups were compared by means of one-way ANOVA test with Duncan’s multiple range post hoc, assuming there were significant differences among them when the statistical comparison gave P < 0.05. All analyses were performed in duplicate or triplicate.
3. Results and Discussion
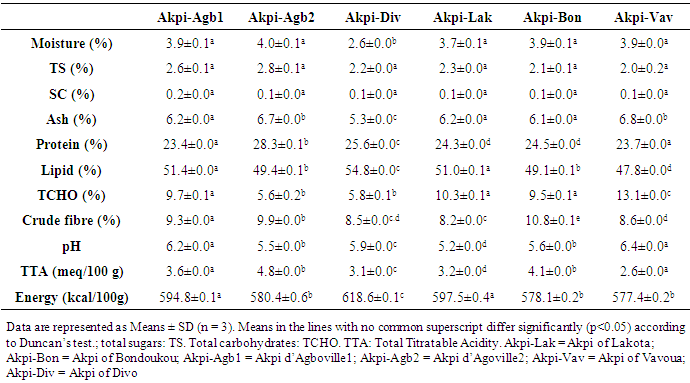
- In this study, the effect of kernels extraction methods on chemical composition and antioxidant activity of flours from R. heudelotti kernels were assessed.Firstly, we performed a nutritional characterization of kernels samples from R. heudelotii differentiating them according to the site of production. The findings were presented in Table 1. Overall, there were significant differences (P < 0.05) between the kernels samples for all the parameters studied. A difference that could be attributed to its steps of preparation (post-harvest traditional technologies) and to the environmental condition. Proximate composition confirm that the seeds kernels from R. heudelotii has potential high lipid and protein content to satisfy calorie and the protein demand of the populations. They contain considerable amounts of proteins (23.4-28.3%), higher than protein rich foods such as quinoa [26], Bambara groundnut [27], cowpeas [28] seeds which ranged between 13.5 - 26.8%. Nevertheless, lipids are a major component of kernels (47.8% - 54.8%). These finding were in agreement with other studies which reported that the lipids content can vary from 47.0 to 60.0% [29, 30]. The high oil content suggests that R. heudelotii can be used as potential source of raw material for commercial activities such as for Millettia ferruginea seed [31]. Furthermore, intrinsic differences between proteins on the one hand and lipids on the other hand (Table 1) may be explained by the compositional changes following the degradation of other constituents during the cooking steps [32]. For the vegetables and legumes for example, pre-cooking step in the production process appears to influence the protein content. Heating before cooking improves the nutritional value and the availability of nutrients [33], which could be the case in this study. The fact that the kernels from Divo's production process came out with the highest content could be due to overcooking (overnight) and the high dry matter content of these kernels. In agreement with Vodouhe et al. [34] works, on leafy vegetables, cooking process causes a sharp increase in the lipid content. This increase in lipid content may be due to the water loss and of dry matter concentration. Total carbohydrates (as calculated by difference) were very low (5.6-13.1%), with low amounts of sugar (2.0-2.7%) irrespective of the production areas considered. Similar levels of carbohydrate were observed in the seeds kernels of R. heudelotii collected at Gagnoa region’s in Cote d’Ivoire [35] or at Yaoundé in Cameroon [29]. It was not surprising since several previous studies have shown that the R. heudelotti seeds are naturally known for being very poor in carbohydrate [35, 5]. Our result thus gives us an indication that the energy source is largely lipids and in some extent protein (through deamination). For total dietary fiber, Lakota was the region with the lowest value (8.2%), but the value of 10.8% (Akpi-Bon) obtained to Bondoukou was higher than that in raw lentils seeds (6.3 g/100 g by fresh weight) [36]. From a nutritionally point of view, the range of fiber content recorded is advantageous as fiber in food is essential in decreasing cholesterol and blood sugar. It absorbs water and provides roughage for the bowels, assisting intestinal transit [37]. The calculated metabolizable energy values of kernels presented in Table 1 ranged from 577.4 kcal/100 g (Akpi-Vav) to 618.6 kcal/100 g (Akpi-Div). These values are close with previous investigations [35, 5] and are greater than those found by CIFOR [33]. The differences observed in these samples could be due to the difference in protein, lipid and fiber reported in proximate composition. Indeed, both lipid (47.8% - 54.8%) and protein level (23.4-28.3%) were the highest contributors to calories from the determination of energy produced. The carbohydrate contents did not considerably affect the determination of energy. The residual moisture content of kernels varied from 2.8% (Akpi-Div) to 4% (Akpi-Agb2) and was slightly lower than what was reported by the literature [29, 35, 7]. For Hong et al. [38], food products with moisture content of 3.1 to 8.7% have water activity less than 0.7. This explain why their seeds kernels can be stored for a long time with minimum chemical degradation and microbial contamination [39]. A significant differences remain (p ≤ 0.05) especially, between the production process of Divo region’s (2.8%) and other regions (3.9% in mean; p ≥ 0.05). A result could be attributed to its preparation steps, such as a solar drying time. He differs from one diagram to another and to the environmental conditions (Figure 2). The ash content of samples ranged from 5.3% (Akpi-Div) to 6.8% (Akpi-Vav). These levels vary from one process to another and are slightly lower with ash values of 7.5% reported by Kouamé et al. [35], but also are closely low with ash values of 3.7%, 3.2% and 3.6% reported for pigeon pea, lima bean and lablab bean, respectively [40]. Ash content is an indicator for mineral elements [31]. Thus, from the result it could be seen that Akpi-Vav sample with 6.8% was the best in terms of mineral content. The minor ash variation in kernels could be due to the geographical origin of the samples, the climatic conditions and the edaphic characteristics of soils [41]. It has been recommended by Pomeranz and Clifto [42] that ash contents of seeds and tubers should be in the range 1.5-3.5% in order to be suitable for animal feeds and human consumption. In this study, the ash content fall within this range hence it can be recommended for animal feeds and human consumption. For all the samples, pH was slightly acid (5.2-6.4), while total acidity was low (2.6 to 4.8 meq / 100 g). This may confer longer keeping quality of them [43]. Total acidity in the samples are consistent with the observations of Saki et al. [5]. These authors reported values between 2.8 - 3.1 meq/g. From the statistical analysis, both total titrable acidity and pH showed that all the samples were significantly different (p ≤ 0.05) and the differences observed may be related to the storage process, which is a function of the drying step [44]. According to Treche et al. [44], the drying time has a significant influence on the acidity of the flour. A long drying time would reduce the titratable acidity of the flours. The determinations of titratable acidity, pH and oxalic acid is of interest because of their alleged adverse effect on mineral bioavailability.
|
|
|
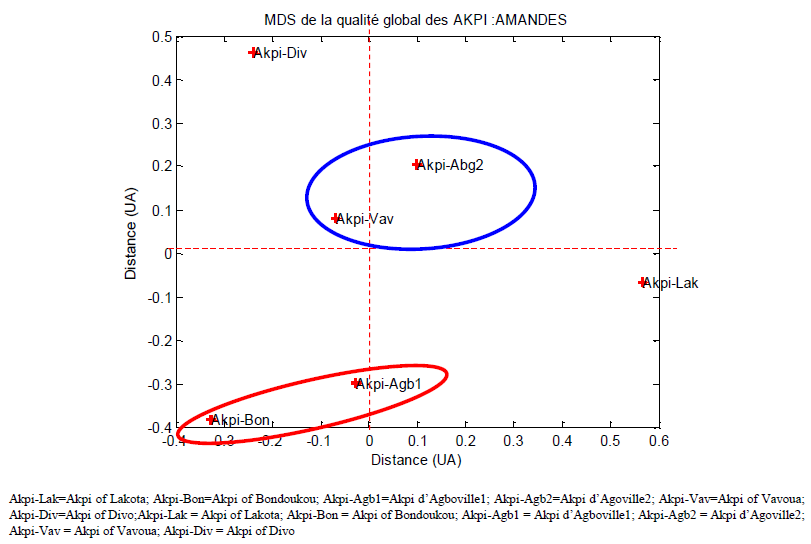
3.1. Multidimensional Scaling (MDS) Analysis
- By performing principal coordinate analysis, also known as multidimensional scaling, the MDS plot presented in Figure 3 was obtained. Multidimensional scaling (MDS) projects a distance matrix into a set of coordinates such that the Euclidean distances of these coordinates approximate the original distances [61]. The distances between the objects depicts their (dis)similarities: similar objects are represented by points that are closer, and dissimilar objects by points that are further apart. The dimensions (Distance UA in figure 3) are factors with real meaning that make it possible to explain the differences among groups. The MDS plot (Figure 3) shows that the R. heudelotii seeds kernels samples were clearly classified into two groups and an individual sample according to seeds kernels extraction systems and physico-chemical characteristics: Agboville1-Bondoukou diagram (Akpi-Bon; Akpi-Agb1, code sample); Agboville2-Vavoua diagram (Akpi-Agb2; Akpi-Vav; code sample) and an individual samples (Divo diagram and Lakota diagram).
4. Conclusions
- The results in the present study reaffirm that seed kernels of R. heudelotii has higher nutrient composition and calorie value compared to some legumes most especially in terms of crude oil and protein. From the result, the kernels extraction techniques employed may influence the physicochemical composition and antioxidant properties of seeds kernels. In particular, the contents of crude protein, crude fat, oxalate, phytate and ash were highly variable. MDS identify the relevant chemical compounds responsible for the main differences among samples and extrinsic factors such as geographical location (locality of production) and kernels extraction systems. These results provide useful indications of the effect of traditional extraction process on the physico-chemical and antioxidant properties of kernels flours from R. heudelotii.
ACKNOWLEDGEMENTS
- This research was performed in partial fulfilment of the requirements for a thesis in Food Sciences and Technology. The authors thank two anonymous reviewers for helpful comments that greatly improved an early version of the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML