-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2018; 8(1): 5-9
doi:10.5923/j.food.20180801.02

Analysis and Comparison of Nutritional, Anti-Nutritional and Mineral Values in Two Kinds of Treculia Perrieri (Tsitindry) Meal comes from Sun Dried Seed and Cooked Seed
Zafilaza Armand, Andriantsimahavandy Abel, Randrianarivo Ranjana Hanitra, Ramamonjisoa Daniel
University of Madagascar Antananarivo, Faculty of the Sciences, Department of Fundamental and Applied Biochemistry, Madagascar
Correspondence to: Zafilaza Armand, University of Madagascar Antananarivo, Faculty of the Sciences, Department of Fundamental and Applied Biochemistry, Madagascar.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

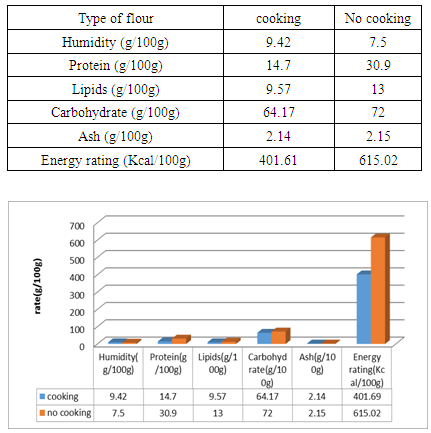
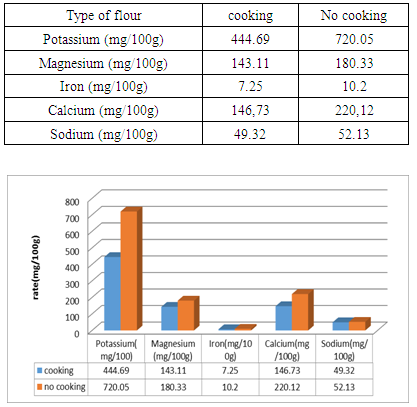
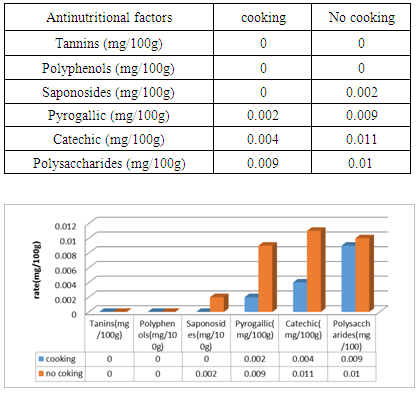
Given the current climate change and rice price spikes in Madagascar, the government must turn to natural products such as Treculia perrieri. The present work was carried out in order to compare the different elements in the flour prepared from cooked and uncooked seed. The levels of lipids, proteins, glucose, ash and energy value in flour made from uncooked seed of 13g/ 100g; 30.9g/ 100g; 72g/100g; 2.15g/100g and 615.02Kcal/100g are superior to those found in the cooked seed flour of 9.42g/100g; 14.7g/100g; 64.17g/100g, 2.14g/100g and 401.61g/100g. Except the water content is greater than 9.42g/100g in flour made from cooked seed compared to uncooked seed flour 7.5g/100g. Similarly for mineral levels in uncooked seed flour such as potassium 720.05g/100g, magnesium 180.33g/100g, iron 10.2g/100g, calcium 220.12g/100g and sodium 52.13g/100g are higher compared to cooked seed flour of 444.69g/100g; 143.11g/100g; 7.25g/100g; 146.73g/100g; 49.32g/100g. In addition the rates of anti-nutritional in cooked flour are inferior to the flour comes from uncooked seed, except the tannin and polyphenol levels are absent in both types of flour. The rates are 0mg/100g for saponins, 0.002mg/100g for pyrogalic, 0.004mg/100g for catechol and 0.009mg/100g for polysaccharides against saponin level is 0.002mg/100g, 0.009mg/100g for pyrogallics, 0.011mg/100g for catechics and 0.01mg/100g for polysaccharides.
Keywords: Treculia perrieri (Tsitindry), Physico-chemical analysis, Nutritional, Antinutritional, Cooked baked flour, Uncooked seed meal
Cite this paper: Zafilaza Armand, Andriantsimahavandy Abel, Randrianarivo Ranjana Hanitra, Ramamonjisoa Daniel, Analysis and Comparison of Nutritional, Anti-Nutritional and Mineral Values in Two Kinds of Treculia Perrieri (Tsitindry) Meal comes from Sun Dried Seed and Cooked Seed, International Journal of Food Science and Nutrition Engineering, Vol. 8 No. 1, 2018, pp. 5-9. doi: 10.5923/j.food.20180801.02.
Article Outline
1. Introduction
- Treculia perrieri are wet woods below 300m, alluvium; it blooms in the months of July and October. The fruits are ripe between the months of January - February. Tsitindry is widely spread in the DIANA region, specifically the district of Ambanja (Sambirano). Treculia perrieri (Tsitindry) is a plant endemic to Madagascar. It is classified in:Class: EquisetopsidaSubclass: MagnoliidaeSuperorder: RosanaeOrder: RosalesFamily: MoraceaeGenus: TreculiaSpecies: Treculia perrieriVariety: Treculia perrieri var. perrieriVernacular names: Katoka, Tobory, Tsipa, Titindry [1-4]Treculia perrieri is a tree up to 30m deep trunk furrowed winged buttresses, bark smooth and greyish. Young pubescent twigs. Leaves persistent petiole puberulous at first, 8 to 12 mm. Leafy blade, angular or obtuse and unequilateral at the base rounded or acute at the apex, wider in the lower half than the upper half, 11 to 18 cm long by 4 to 7 cm; About 15 lateral veins on each side; thin nerves regularly crosslinked. Dioecious flowers rarely monoecious, usually male receptacles on young twigs, females on aged twigs [1-4].Receptacles male, obovate with shrunken base, up to 4cm by 3. Flowers intertwined with peltate bracts in escutcheon, welded on the 2/3 of their length, exceeded by the flowers at anthesis. Perianth hyaline short-bellied with 3-4 small ciliate teeth and 4 exserted stamens. Female receptacles of similar shape, but larger (6 out of 5). Female flowers in several rows, interspersed with peltate bracts, the stigmata protrude alone. Two stigmatic branches of 5 to 7mm slightly papilleuses, obtuse, surmounting a hairy style of about 3mm. Syncarp sessile irregular shape exceeding 30 cm and 5 kilos, fleshy, presenting towards the surface 6-7 rows of ovoid achenes about 1 cm. Woody pericarp slender, brown on dry seed without albumen; thin integument. Unequal cotyledons completely folded the notched, bilobed tops receding at the level of the radicle, the widest enveloping the other in tongue [1-4].
2. Methods and Materials
2.1. Preparation of Treculia perrieri Flour
- a) Preparation of Treculia perrieri flour after cooking: the seeds are extracted from ripe fruit. And after, the seeds are separated from the pods and boiled for a few moments so that the pod around them bursts. Drain and dry the pods before shelling them for ease. After the cotyledons are sun-dried then the cotyledons are ground to obtain the Treculia perrieri flour.b) Preparation of Treculia perrieri flour after drying in the sun: after extracting the seeds from ripe fruits, the seeds are washed and immediately dried in the sun to burst the pods. Shelled sun-dried seeds and cotyledons before grinding for flour.
2.2. Analyzes of Minerals in Two Types of Flour
- The flour is put into a muffle oven at 550°C to obtain a white ash containing the minerals.The Ca, Mg, K, Na mineral contents are determined by atomic absorption spectrophotometry. After wetting, 5 to 25 ml of concentrated hydrochloric acid is added. The suspension is then boiled and filtered. The phosphorus level is determined by colorimetry or spectrophotometry at 560 nm [6, 9, 13, 26].
2.3. Nutritional Value Analysis in Two Types of Flour
- - Lipids: the sample is treated with hexane. Five grams of sample are introduced into extraction cartridges for six hours. The extracted extract is put in a drying oven at 75°C for one hour until a constant mass is obtained [26, 32, 35, 43].- Crude ash: the 5 g sample taken is placed in a muffle furnace set at 550°C. White ash is weighed after cooling.- The proteins:Two protein extraction techniques are used.a) The flour obtained is suspended in a sodium phosphate buffer (0.05 mol.l-1 at pH 8.0) at a rate of 4 g in 9 ml. The debris is removed by centrifugation at 20,000 g. Proteins from the supernatant and the centrifugation pellet is precipitated in the presence of TCA at 50 μl-1. They are dissolved in decinormal soda and measured according to the LOWRY method [6, 11, 26].b) The flour is suspended in a sodium phosphate buffer (1 mol.l-1 at pH 0.8) at a rate of 1 g in 9 ml and milled under constant pressure (420 kg/cm2).Cell debris is removed and the suspension centrifuged at high speed under the conditions described in the art. The soluble proteins obtained, according to the two techniques, are separated into two groups by chromatography and the supernatants extracted after high speed centrifugation are dialyzed for 16 hours against a sodium phosphate tap (0.01 mol.l-1 at pH 8, 0) containing urea at a final concentration of 8 mole.l-1. The dialysates are then chromatographed in a column of diethylaminoethylcellulose equilibrated with the same buffer. In this method, the cationic protein retained by the resin and in other cases the anionic proteins remain adsorbed. The cationic and anionic proteins separated by chromatography are hydrolysed at 140°C. for 24 h in the presence of 6N HCl. Their respective amino acid composition is determined after analysis of the hydrolysates [6, 11, 26].- Carbohydrates: are determined spectrophotometrically at 490 nm. The Fischer and Stein method is applied and it uses DNS at 540 nm to evaluate soluble sugars [14, 18, 24, 26].
2.4. Determination of Antinutritional Factors in Two Types of Treculia perrieri "Tsitindry" Flour
- The determination of the total phenol content was based on the reaction with the Folin Ciocalteu reagent. The blue color obtained has a maximum absorption at 725 nm. The tannins were determined according to the spectrophotometric method using acidified vanillin and tannic acid as standard (λmax = 500 nm). Determination of saponin content was made using the aerosimetric method based on the formation of stable foams by Koziol saponins [20, 25, 26, 28, 33].
3. Results and Discussions
3.1. Nutritional Value of Treculia perrieri "Tsitindry"
|
3.2. Minerals in the Treculia perrieri "Tsitindry"
- a) Potassium, magnesium, iron, calcium and sodium minerals:- Potassium level. 720.05mg/100g the potassium level in the uncooked seed against 444.69mg/100g of flour from the cooked seed. The potassium level in the cooked seed meal is half of the potassium level in the uncooked seed meal. So the potassium level in the uncooked seed meal is much higher compared to the cooked seed. The heat removes 1/2 of potassium levels in the seed. The seed of "Tsitindry" is very rich in potassium.- Magnesium levels. The seed of Treculia perrieri contains magnesium with an interesting rate. But, the rate in uncooked seed meal is higher than that of cooked seed flour 180.33mg/100g against 143.11mg/100g. Magnesium plays a role, important in human life, it is very interested to eat flour comes from uncooked seed for assimilated maximum magnesium.- Real iron. Iron is among the trace elements in the human nutrient. Treculia perrieri shows that in flour prepared with uncooked seed at a rate of 10.2mg/100g greater than the rate of cooked seed flour 7.25mg/100g. The heat destroys some iron levels in the cooked flour.- Late of calcium. 220.12mg/100g calcium level in uncooked seed flour against 146.73mg/100g cooked seed flour. The result shows that the calcium level in the uncooked seed meal is higher than that in the cooked seed meal. But the heat destroys 73.39mg/100g of calcium in the seed.- Sodium level. Sodium level 49.32mg/100g for cooked seed against 52.13mg/100g of cooked seed. Sodium has a mean level in the seed of "Tsitindry".Based on the results, mineral levels in uncooked seed meal are high compared to flour made from cooked seed. This causes intense heat during the cooking of seed. Volatile elements are very sensitive to heat as Calcium almost moist rates are gone. Similarly for potassium, magnesium.
|
3.3. Antinutritional Value in Treculia perrieri "Tsitindry"
|
4. Conclusions
- It is difficult to distinguish in which type of flour is very important in food. The results show that the presence of heat decreases the rate of anti-nutrients, mineral levels and nutritional values in flour prepared from cooked sheath. To conclude that mineral rates and nutritional values in uncooked seed are almost twice the rates in the cooked seed. The flour of "Tsitindry" is an effective relief to solve the famine in Madagascar.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML