-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Food Science and Nutrition Engineering
p-ISSN: 2166-5168 e-ISSN: 2166-5192
2017; 7(5): 119-124
doi:10.5923/j.food.20170705.03

Nutritive Assessment of the Fruit Pulp of Coelocaryon oxycarpum, an Aromatic Plant Consumed as Spice
Josi-Noelline Sinh1, Jean Tia Gonnety1, Jean Didier Kouassi-Koffi1, Chépo Ghislaine Dan1, Doudjo Noufou Ouattara2, Adama Bakayoko2
1Training and Research Unit in Food Sciences and Technology, Nangui Abrogoua University, Côte d’Ivoire
2Training and Research Unit in the Natural Sciences, Nangui Abrogoua University, Côte d’Ivoire
Correspondence to: Jean Tia Gonnety, Training and Research Unit in Food Sciences and Technology, Nangui Abrogoua University, Côte d’Ivoire.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The fruit pulp of Coelocaryon oxycarpum (Cox), an aromatic plant, is consumed as a spice in the eastern part of Côte d’Ivoire. This study aimed to evaluate the physicochemical as well as the nutritive properties of Cox fruit. The fruit pulp powder showed relatively high level of proteins (16.16% dm), crude fiber (46.98% dm), carbohydrate (24.20% dm), vitamin B6 (47.68 mg/ 100 g dm), vitamin B2 (33.91 mg/100 g dm), ash (5.09% dm), but low fat (7.57 % dm) and energy (189.19 Kcal/100 g dm). Mineral composition (mg /100 g, dm) revealed that potassium (1566.71±47.15) was the most abundant mineral followed by Mg (126.79), calcium (124.21) and phosphorus (113.9). It contained also appreciable amount of iron (21.68± 7.26). Amino acid profile (mg / 100 g, dm) showed proline (654.73), valine (314.82) and leucine (141.22) as major amino acids. Therefore, the dried pulp of Cox fruit could be considered as a potential source of nutrients and may be of use in food industries as a source of ingredients with high nutritional value.
Keywords: Coelocaryon oxycarpum, Fruit pulp, Nutritive value, Spice
Cite this paper: Josi-Noelline Sinh, Jean Tia Gonnety, Jean Didier Kouassi-Koffi, Chépo Ghislaine Dan, Doudjo Noufou Ouattara, Adama Bakayoko, Nutritive Assessment of the Fruit Pulp of Coelocaryon oxycarpum, an Aromatic Plant Consumed as Spice, International Journal of Food Science and Nutrition Engineering, Vol. 7 No. 5, 2017, pp. 119-124. doi: 10.5923/j.food.20170705.03.
Article Outline
1. Introduction
- Wild edible plants are an important part of local food systems. They are generally used as sources of nutrients, but also as a medicine for the treatment of certain diseases. These plants contribute substantially to food by ensuring the survival and providing nutrients in the diet. They provide food in the form of fruits, leaves, roots, seeds and other plant parts, either raw or cooked [1]. Trade in edible parts of wild plants, generally held by women in Africa, improves the incomes of many low-income households [2]. The use of these plants is based on the know-how and empirical know- ledge of some populations. They are thus ignored by a large part of the population because data that can explain the virtues attributed to them are scarce or non-existent [3]. Thus, a nutritional and functional characterization of these plants could allow a better exploitation and valorization of their nutritional and curative potential.Coelocaryon oxycarpum (Cox) is an edible aromatic plant found in the eastern part of Cote d'Ivoire. This plant belongs to family Myristicaceae. The genus Coelocaryon comprises five species (Coelocaryon pressii or multiforme Warb; Coelocaryon oxycarpum Stapf, Coelocaryon sphae- rocarpum Fouilloy; Coelocaryon botryoides Vermoesen; and Coelocaryon klainei Pierre) [4, 5] that are endemic to African regions.The food use of fruits of Cox has been reported for the first time by Ouattara et al. [3]. Burkill [6] reported using its sap in traditional medicine as a laxative, but makes no reference to the dietary use of its fruits. Cox fruit has a high socio-cultural value in the region where it is consumed [3]. Indeed, the fruits of the plant, that mature during the month of March and available until the end of June, are picked up and sun-dried by women during this period. The dried fruits are then preserved either for domestic use or sale [3]. These dried fruits constitute a spice well appreciated by the people who know it. They are used preferentially for the preparation of the sauces (soups) for women who have just given birth. According to the users, the consumption of the sauce made with its fruits allows a total rejection of blood clots after the childbirth and a rapid healing of the wounds [3]. To date, no specific studies evaluating the phytochemical compounds playing these roles have been carried out on this plant. Note that, local foods and neglected foods are characterized not only by local importance; they also might have the potential to improve diet diversity on a more supra-regional level [7].The objective of this study was to assess the nutritive composition of the dried pulp of Cox fruit.
2. Materials and Methods
2.1. Sample Collection and Preparation
- The plant material used in this study is the fruit pulp powder of Cox. The fruits were harvested in the department of Bondoukou, precisely in the sub-prefecture of Laoudigan in the North-East of Côte d’Ivoire. Once harvested, fruits (Figure 1) were placed in ice box to keep them fresh and transported to the laboratory for future analyses. Then, to obtain the powder, two (2) kg of fresh fruits were pitted, washed with distilled water, drained, grated and dried in an oven at 45°C for 72 h. After drying, the fruit pulps were crushed using a Moulinex-type mixer. The powder obtained was sieved (250 μm) to obtain the fruit pulp powder. The powder was put into boxes and stored in a cool dry place.
 | Figure 1. Photographs of sheets and fruits of Coelocaryon oxycarpum, A: sheets and fruits, B: pulp of fresh fruit |
2.2. Proximate Analysis
- Moisture, ash, crude fiber, crude protein, lipid and carbohydrate contents were determined in accordance with the standard methods of the AOAC [8]. All determinations were run in triplicates. The moisture content was deter- mined by the difference of weight before and after drying the sample (10 g) in an oven (Memmert, Germany) at 105°C until constant weight at least or 72 h. Ash fraction was determined by the incineration of dried sample (5 g) in a muffle furnace (Pyrolabo, France) at 550°C for 12 h. The percentage residue weight was expressed as ash content. Fiber estimate was obtained from the loss in weight of dried residue following the digestion for fat-free samples with 1.25% each of sulfuric acid and sodium hydroxide solutions. The crude protein content (N × 6.25) was estimated by the macro-kjeldahl nitrogen assay method using a digestion apparatus. The fat content was determined by Soxhlet extraction using hexane as a solvent. Reducing sugar content was determined by extracting with 80% neutral aqueous ethanol followed by evaporation of the ethanol and subsequent measurement using the dinitrosalicylic acid method according to Bernfeld [9]. Total soluble sugar content in ethanolic extract was assessed using the phenol-sulfuric acid method according to Dubois et al. [10].Carbohydrate and calorific values were calculated using the following formulas [11]:Carbohydrates (dry matter basis): 100- (% moisture + % proteins + % lipids + % ash + % fibers).Calorific value (dry matter basis): (% proteins × 2.44) + (% carbohydrates × 3.57) + (% lipids × 8.37). The results of ash, fiber, protein, fat and carbohydrate contents were expressed on dry matter basis.
2.3. Minerals Analysis
- The mineral elements were analyzed after wet-ashing using the scanning electron microscope (SEM) with variable pressure (SEM FEG Zeiss Supra 40 VP) according to AOAC [12]. This SEM is equipped with an X-ray detector (Oxford Instruments) connected to an energy diffusion spectrometry (EDS) micro-analyzer platform (Inca Cool Dry, without liquid nitrogen). About 10 mg of the sample ash residue were applied evenly to a primed platform with double-sided adhesive carbon for analysis. To measure the content of chemical elements, the device performs a measurement of the transition energy of the electrons from electronic clouds of the K, L and M series of atoms of the sample.
2.4. Amino Acids Determination
- Amino acid was determined using the method described by Deguine and Hau [13]. Amino acids were assayed by reverse phase high performance liquid chromatography (PTC column RP-18, 220 mm in length, 2.1 mm internal diameter) equipped with a pre-column (SHIMADZU SPD 20A, Japan). The sample was vacuum hydrolyzed at 150°C for 60 min in a Waters Pico-Tag work station (Waters’ associates, Milford, MA, USA) in grade 6 N HCl at 1% phenol. It was then taken up in ultra-pure water and derivatized automatically by means of an auto-derivator-analyzer-420 (SHIMADZU SPD 20A, Japan). The amino acid derivatives in the form of phenyl isothiocyanates (PITC-amino acids) were separated by chromatography system consisted of mobile phase A (45 M sodium acetate, pH 5.9) and mobile phase B (30% 105 mM sodium acetate pH 4.6; 70% acetonitrile) with a linear gradient of 7-36% at 1.5 mL/min. The detection was done at a wave- length of 254 nm and the total duration of the analysis was 31 min. The acquisition and exploitation of the results were carried out using the Model 600 Data Analysis System (SHIMADZU SPD 20A, Japan) software.
2.5. Organic Acids Determination
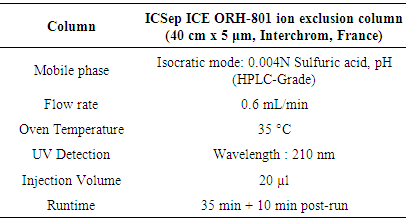
- Organic acids were extracted from 1 g of sample with 50 mL of 80 % methanol saturated with NaCl and were analyzed according to the method of Karadeniz [14] using a HPLC system (Shimadzu Corporation, Japan) consisting of a pump (Shimadzu LC-6A Liquid Chromatograph, Japan), a UV detector (Shimadzu SPD-6A UV Spectrophotometric de- tector, Japan) and an integrator (Shimadzu CR 6A Chromatopac, Japan). All separations were carried out in isocratic mode with an ICSep ICE ORH-801 ion exclusion column (40 cm x 5 μm, Interchrom, France) maintained at 35°C using a Meta ThermTM furnace (Interchrom, France).The HPLC conditions are shown in the Table 1. Organic acid standards (citric, fumaric, malic, oxalic, tartaric acid, quinic acid, ascorbic acid, succinic acid and fumaric acid) were obtained from Aldrich Co. (Sigma-Aldrich Chemie, Steinheim, Germany). The standard solutions were prepared individually at different concentrations with double distilled water. The analysis was carried out in triplicate. The levels of the organic acids in the samples is obtained by comparing the retention times of the eluted compounds with the retention times of the reference solutions.
|
2.6. Determination of B vitamins
- B vitamins were determined according to AOAC [12] method. A 5 g of liquid/powder sample was extracted with 20 mL of methanol (80%). The stock of standard (Sigma-Aldrich) was prepared by dissolving 0.01 g of each standard in methanol (80%). A HPLC system (SHIMADZU SPD 20A) equipped with UV detector (PAD) and C18 ODS column (250 x 4.6 de, Cluzeau France) was used in isocratic mode for analysis. Mobile phase consisted of acetonitrile (55 mL), tetrahydrofurane (37 mL) and water (8 mL) at 1.5 mL/min flow rate and 10 µl of each sample/standard were injected and monitored at 325 nm wavelength.
2.7. Statistical Analysis
- All analyzes were carried out in triplicate. Results were expressed as mean values ± standard deviation (SD).
3. Results
3.1. Proximate Composition
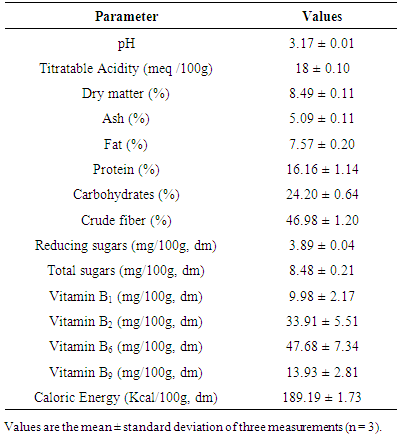
- The chemical composition and energy value of Cox fruit are presented in Table 2. The pH and titratable acidity values were 3.17 and 18 meq/100 g, respectively. The dry matter (8.49%), ash (5.09%, dm), fat (7.57%, dm) contents were low, while protein (16.16%, dm), carbohydrates (24.20%, dm), crude fiber (46.98%, dm) contents were relatively high. Reducing sugars and total sugars values (mg/ 100 g, dm) were about 3.89 and 8.48, respectively. The vitamin compositions (mg /100g, dm) were as follows: vitamin B1 (9.98), B2 (33.91dm) B6 (47.68) and B9 (13.93). The energy value (kcal/ 100g, dm) was 189.19.
|
3.2. Amino Acid Contents
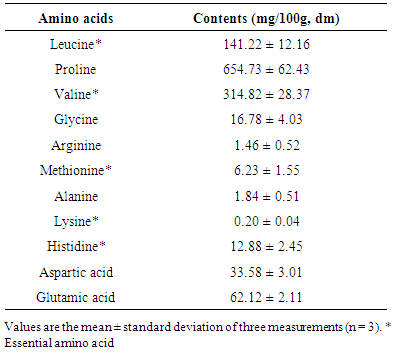
- About ten (10) amino acids were detected in the dried pulp powder of Cox fruit with a predominance of proline (654.73 ±62.43 mg/100g dm) and valine (314.82 ± 28.37 mg/100g, dm). It was also noticed that 5 of these compounds were essential amino acids: valine, leucine (141.22±12.16 mg/100g, dm), methionine (6.23 ±1.55 mg/100g, dm), alanine (1.84 ±0.51 mg/100g, dm), and a low lysine (0.20 ±0.04 mg/100g, dm) content (Table 3).
|
3.3. Mineral Contents
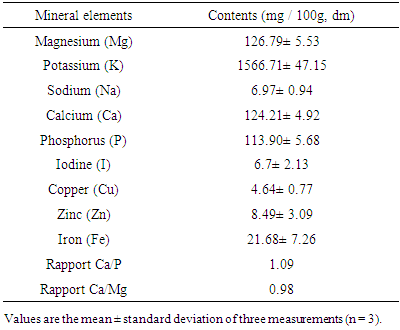
- Table 4 shows the mineral composition of the dried pulp of Cox fruit. The levels of Na, K, Ca, P and Mg (mg/100 g, dm) were 6.97±0.9, 1566.71±47.15, 124.21±4.92, 113.90±5.68 and 126.79±5.53, respectively. Those of trace elements, in particular Fe, Cu, Zn and I (mg/100 g, dm), were 21.68±7.26, 4.64±0.77, 8.49±3.09 and 6.7±2.13, respectively.
|
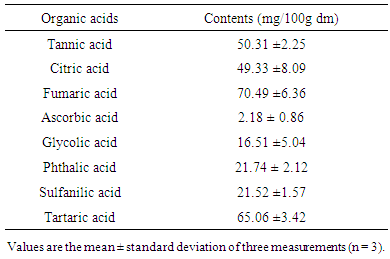
3.4. Organic Acid Contents
- Organic acids profile of the dried powder of Cox fruit is listed in able 5, it was observed appreciable levels (mg / 100g, dm) of fumaric acid (70.49 ±6.36) and tartaric acid (65.06 ±3.42) and slightly less tannic acid (50.31 ±2.25), citric acid (49.33 ±8.09), ascorbic acid (2.18 ± 0.86) glycolic acid (16.51 ±5.04), phthalic acid (21.74 ± 2.12) and sulfanilic acid 21.52 ±1.57).
|
4. Discussion
- Proximate composition revealed that fresh pulp from fruits of Cox contained about 91.51% moisture and 8.49% dry mater. This high moisture content indicated that Cox fruits are highly perishable. Indeed, it is well established that high moisture content of food products promotes susceptibility to microbial growth and enzyme activity which accelerates spoilage [15].Ash content of the fruit pulp from Cox was 5.09 ± 0.11%. This ash level (g / 100g, dm) appeared lower than that of spices such as Monodora myristica (9.6 ± 0.1), and Xylopiaa ethiopica (9.5 ± 0.1) [16]. However, regarding its mineral profile, the dried fruit pulp can be considered as a good source of K, P, Mg and Fe. It contains more K (1566.71 ± 47.15 mg / 100g dm) than Achillea millefolium (1267 ± 10 mg/100g dm), but low than Taraxacum sect. Ruderalia (2851 ± 52 mg/100 g dm) [17], which are wild edible plants. The richness of Cox fruit in K is of great importance. In fact, it has been reported that K regulates the heartbeat [18]. It is an important mineral useful for muscular contraction and maintaining electrolyte balance in humans. K is also an important factor in regulating blood pressure and decreasing the risk of cardiovascular disease [18]. About 225 g of fruit pulp of Cox could cover the daily requirement in K of an adult (3510 mg/day) [19], so that it regular consumption may regulate blood pressure. As regards Fe, it content (21.68±7.26 mg / 100 g dm) was found to be higher than those of some African leafy vegetable such as Cucurbita maxima Duchesne (9.2 mg/100 g dm), Citrullus lanatus Thunb. (6.4 mg/100 g dm) [20], but closed to those of fresh leaves (21.72 ± 0.61 mg/100 g dm) and flower (23.34 ± 0.12 mg/100 g dm), dried leaves powder (18.86 ± 1.20 mg/100 g dm) of Moringa oleifera [21]. Although the bioavailability of Fe in plant products is lower than in animal products, the consumption of fruit pulp of Cox by women may be particularly useful because Fe deficiency is a major problem among pregnant women in developing countries [22]. According to WHO/FAO, [19], Fe requirements for adults are estimated to be between 6 and 7 mg / day, and Zn (7.5-11mg/day). Thus the daily consumption of 30-130 g of Cox fruit pulp in sauce or in powder could cover the daily requirement of Fe and Zn, respectively. The rate of Cu and Mg in the fruit pulp could also be beneficial for consumers because these minerals are likely to increase the absorption of vitamin B12 [23].The fruit pulp appeared to be rich in B group vitamins. Thiamine or vitamin B1 is a coenzyme in the pentose phosphate pathway, which is a necessary step in the synthesis of fatty acids, steroids, nucleic acids and the aromatic amino acid precursors to a range of neurotransmitters and other bioactive compounds essential for brain function [24]. Vitamin B2 is essential for energy production. It also plays an indirect role of antioxidant, while vitamin B9 helps to meet protein needs, synthesizing chemical messengers of the brain and nucleic acids for DNA and RNA. The protein content of Cox (16.16%) was higher than those obtained by Abdou [25] in M. myristica (13.61 g / 100g dm) and D. Psilurus (4.82 g / 100g dm). B group vitamin contents together with protein level would support the assertion that the fruit consumption as spice or vegetable would allow the fortification of the women after the childbirth [3]. In addition to that, the amino acid profile of fruit pulp of Cox showed interesting amounts of essential amino acids like valine (314.82 ± 28.37 mg/100 g dm) and leucine (141.22±12.16 mg/100g dm).As concern lipid, it should be noted that the relatively low value in fruit samples corroborate the findings of many authors who showed that vegetables are poor sources of fat [26]. The estimated calorific values (189.19 ± 1.73 Kcal / 100 g dm) agreed with general observation that vegetables have low energy values due to their low crude fat and relatively high level of moisture [27]. However, it’s important to note that diet providing 1-2 % of its caloric energy as fat is said to be sufficient to human beings, as excess of fat consumption yields to cardiovascular disorders such as atherosclerosis, cancer and aging [28]. The rate of crude fiber makes the fruit pulp as fiber-rich food. Several studies have shown that consuming fiber-rich food would be an advantage for overweight people [29]. Therefore, the consumption of the fruit pulp of Cox in large amount may be recommended to individuals suffering from obesity.The titratable acidity of the fruit pulp powder was about (18 meq / 100g dm). This acidity very low compared to that of Adansonia madagascariensis (95 meq / 100g) [30], is probably due to presence of various organic acids such as fumaric acid, tartaric acid, tannic acid, citric acid, glycolic acid, phthalic acid, and sulfanilic as revealed by the analysis. The subsequent low pH of the fruit pulp (3.17) is likely to favor the development of yeasts and molds [31]. Thus, drying could be one of the best ways of processing the fruit pulp of Cox in order to prevent it from damaging and render the product available over time. Note that, the fruit pulp is used dried as it is very fragrant and very pleasant in dried form. Development of the flavor takes place during the drying process. This flavor would be due partly to organic acids. Indeed, organic acids are known to influence the taste and flavor of fruits and drying also enhances the compounds responsible of the flavor [32]. In addition to that, the presence of these organic acids could benefit consumers of Cox fruit because one of the roles of organic acids is to lower the pH in the stomach, thus reducing the growth of certain pathogenic bacteria [33, 35]. They also promote the availability of nutrients in the diet, improving their digestion, absorption, and retention [36].
5. Conclusions
- The nutritive value of fruit pulp of Cox revealed its useful- ness. The fruit is characterized by relatively high level of proteins, crude fiber, carbohydrate, and vitamin B contents. It can be considered as K-rich food. Therefore, the fruit of this aromatic plant could be considered as a potential source of nutrients and may be of use in food industries as a source of ingredients with high nutritional value. However, due to the high moisture content of the fruit and its low pH, the pulp should be properly dried to prevent it for damaging.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML